Use of carbon monoxide breath test to assess red blood cell lifespan…
Use of carbon monoxide breath test to assess red blood cell lifespan in newly diagnosed multiple myeloma patients
Yanfang Wang, Zhenhao Zhang, Yan Liu, Hongmei Jing, Xiaoyan Ke and Fei Dong
Published 18 July 2019 • © 2019 IOP Publishing Ltd
Abstract
To clarify the role of red blood cell (RBC) lifespan in anemia of multiple myeloma (MM), RBC lifespan was detected in 40 newly diagnosed MM patients by measuring exhaled endogenous carbon monoxide concentration. Mean RBC lifespan was significantly reduced in MM patients (63 ± 23 d) than healthy controls (116 ± 17 d). RBC lifespan in MM patients without anemia (78 ± 21 d) was also significantly lower than for healthy controls. RBC lifespan in MM patients with anemia (52 ± 18 d) was significantly lower than those without. Besides, RBC lifespan in MM patients with renal insufficiency (50 ± 16 d) was lower than those without (66 ± 23 d). RBC lifespan was significantly negatively correlated with % reticulocytes, erythropoietin concentration, % clonal plasma cells, β2-microglobulin (MG) level, and creatinine level. Receiver operator characteristics curve was used to determine a cut-off point (61 d) to predict effect of RBC lifespan on chemotherapy. Overall response rates were significantly higher in MM patients with RBC lifespan ≥61 d than those <61 d. In conclusion, RBC lifespan of MM patients is reduced, and severely shortened RBC lifespan may be involved in the occurrence of anemia in MM patients. The chemotherapeutic effect of MM patients can be predicted by RBC lifespan.
1.Introduction
Multiple myeloma(MM) is a common plasma cell malignancy and accounts for approximately 10%of all hematologic cancers [1, 2]. The median age of patients at diagnosis is approximately 65 years. The clinical and biological manifestations of MM are heterogeneous however, anemia is one of the most common compli-cations, in approximately 75% of newly diagnosed MM Patients [3, 4]. Anemia often contributes to poorer outcomes and reduced survival [5, 6]. Primary mechanisms underlying anemia in MM include the infiltration of myeloma cells into bone marrow nhibition of red blood cell(rBC) hematopoiesis by multiple cytokines(including interleukin-6 [IL-6]tumor necrosis factor-C ITNF-ab and interleukin-1 [IL-1D), erythropoietin(EPo)deficiency (in patients with renal insufficiency or chronic renal disease), impaired iron utilization due to the chronic inflamma-tion, and erythroblast apoptosis induced by myeloma cells [7-10]. Furthermore, shortened RBC lifespan has also been proven to aggravate anemia during chronic nfection chronic leukemia, and other cancers through macrophage activation and accelerated RBC destruction [11-13]. The role of erythrocyte lifespan in mm-related anemia remains unclear, and tradi tional methods for RBC lifespan evaluation such as measurement of RBC labeled with N-glycine,Cr or biotin, are technically complicated time consum ing, and involve exposure to radiation [14-16]. In 1992, Levitt's carbon monoxide(co) breath test was reported for the quantitative assessment of RBC survival status, based on exhaled alveolar endogenous CO and blood hemoglobin(Hb)concentration [17] Endogenous CO in exhaled breath arises mainly from erythrocyte destruction. During Hb degradation, heme can be converted to bilirubin, releasing CO Therefore, expired CO is a useful parameter for the assessment of changes in RBC turnover[18, 19]. In this study, we employed a novel automated sys tem for the assessment of RBC lifespan in newly diagnosed MM patients, based on the principle of Levitts CO breath test. This system has previously been used to detect RBC survival in 109 healthy subjects and its reliability has been validated [20]. The aim of this study was to determine the effectiveness of this new system for evaluating changes of RBC lifespan in MM patients, and to clarify the role of rBC lifespan in the pathogenesis of anemia in MM patients and its effect on treatment.
2. Materials and methods
2. 1. Subjects
Forty newly diagnosed MM patients attending Peking University Third Hospital were enrolled in this study All patients were non-smokers. The diagnosis of MM was made based on the international myeloma Work ing Group(IMwG)2014 diagnosis criteria [21]. In the present study, Hb <10gdI-1 was defined as anemia and creatinine >177 moll-1 was defined as renal insufficiency according to MM-defining CRAB fea-tures(increased calcium, renal dysfunction, anemia and bone lesions)[21]. Data collected from patients comprised sex, age, stage, Ig isotype, and laboratory values including Hb concentration, EPO concent ration, reticulocyte percentage, β2 microglobulin (β2-MG)levels, and creatinine levels. In addition, 10 non-smoking patients diagnosed with autoimmune hemolytic anemia(AIHA)were included in the study as positive controls, and 10 non-smoking healthy volun teers with normal Hb concentrations and no history o heart, hepatic, renal or pulmonary diseases,were included as negative controls. This study(registration no. M2019010)was approved by the institutional review board of the Peking university Third Hospital and informed consent was obtained from allsubject.
2.2. Sample collection
Alveolar air samples were obtained according to manufacturers instructions(ELS TESTER, Seekya Biological technology Co, LTD, Shenzhen, China). All subjects fasted in the morning preceding sample collection. In brief, breath collection apparatus was placed into the subject's mouth, subjects breathed deeply and held their breath for 10 s, then exhaled into the collection system. The collection system automati-cally discarded the first 300 ml exhaled air and collected the subsequent alveolar air into a self-sealin foil bag. If required, the procedure was repeated until 1 l of alveolar air had been collected. Simultaneously, atmospheric samples were collected into self-sealing oil bags using an electric air pump. Peripheral vein blood samples for routine Hb measurement were collected on the same day as alveolar air sampling.
2.3. RBC survival analysis
RBC survival was measured using a commercial erythro-cyte lifespan assessment instrument(ELS TESTER, Seekya Biological technology Co, LTD, Shenzhen, China). In brief, foil bags filled with alveolar and environmental air were connected to their appropriate positions on the instrument. Concentration of endogenous co was calculated as the difference between alveolar co and background environmental CO. After inputting Hb value obtained from laboratory test and triggering the start button, RBC survival was automatically calculated Ing from endogenous Co and Hb concentration, accordin to the simplified Levitts formula: RBC lifespan (days)=1380 x [Hb/endogenous CO[20].
2.4. Statistical analysis
RBClifespan and other clinical values for subjects were expressed as means t standard deviation. Data were compared between groups using independent t-test Correlation between RBC lifespan and other clinical parameters was calculated using Pearsons correlation coefficient. Categorical variable data were analyzed using Chi-square test. Receiver operator characteris tics(roc) curve was used to determine the rbc lifespan cut-off value for predicting efficacy of che motherapy. a P value <0.05 was considered statisti cally significant.
3. Results
3. 1. Clinical characteristics of subjects
Clinical characteristics of 40 MM patients, 10 AIHA patients, and 10 healthy controls were shown in table 1 The mean age of the three groups was 64, 59 and 56 years old, respectively. Of the 40 MM patients, 23 (57.5%)presented with anemia,8(20.5%) presented with renal insufficiency, and 7(17.5%)presented with simultaneous anemia and renal insufficiency Besides mean reticulocytes and mean EPO level of MM patients were significantly higher than that of health controls(P=0.0046 and P<00001, respectively) but significantly lower than that of AIHA patients
(P< 0.0001 and P=0.0491, respectively).
3. 2. RBClifespan in MM patients
Scatter plots for RBC lifespan in MM patients, AIHA patients, and healthy controls were shown in figure 1(a). Mean RBC lifespan of MM patient 63 23 d)was significantly lower(P< 00001)than that of healthy controls(116 17 d), but significantly higher (P=0.0483) than that of AIHa patients (46+ 24 d) Mean RBC lifespan of non-anemic MM patients(78 t 21 d) was also significantly lower than that of healthy controls(P= 0.0002). Furthermore, RBC lifespan of MM patients with anemia(52+ 18 d) was significant lower(p=0.0002)than those without anemia(figure 1(b). There was no statistical differ ence in RBC lifespan between anemic MM patients and AIHA patients(P=0.4524), but RBC lifespan of non-anemic MM patients was significantly higher than that of AIha patients(P= 0.0014) Mean RBC lifespan of MM patients with renal insufficiency was lower(50±16d) than those without(66±23d) (P=0.0754).
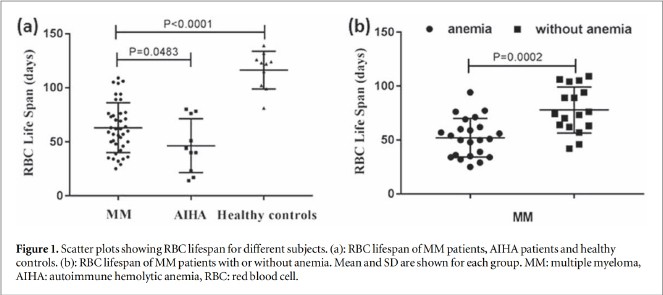
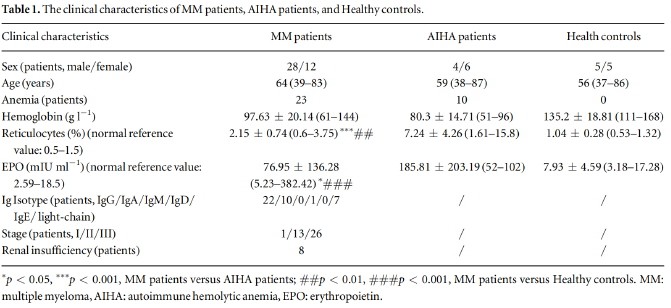
3.3. Correlation between RBClifespan and clinical
parameters
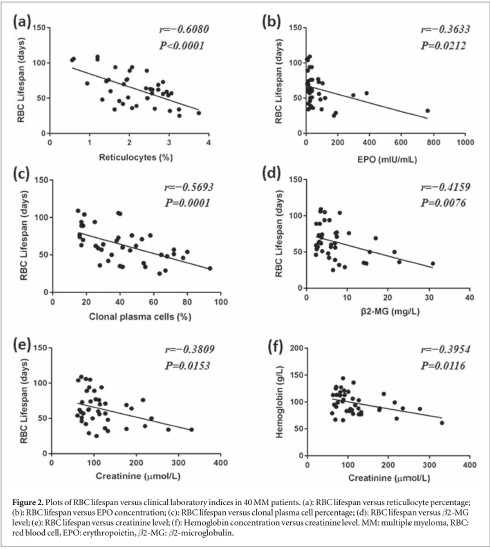
The results of correlation analysis showed that RBC lifespan was not affected by sex, age, m protein or LDH (data not shown), but was significantly negatively correlated with reticulocyte percentage, EPO concen tration, clonal plasma cell percentage and 52-MG level (P<0.05)(figures 2(ad)). The degree of renal insufficiency, as indicated by serum creatinine level, was significantly negatively correlated with both RBC life span and Hb concentration(P<0.05)(figures 2(e)-(D). All of the correlation coefficients(r)and Pvalues of RBC lifespan versus the important clinical parameters were showed in figure 2.
3.4. Effect of RBC lifespan on treatment ofMM
patients
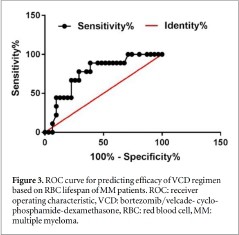
After induction chemotherapy with four cycles of VCD(bortezomib/velcade- cyclophosphamide dexamethasone), overall remission rate(ORR), defined as greater than or equal to partial remission(Pr),was 77.5%(31/40). Depending on whether the patients was remission or not the role of RBC lifespan values in predicting the efficacy of VCD regimen was analyzed by ROC curve. The cut-off value of RBC lifespan was 61 d and the area under the curve for the ROC curve was 0.7599(95%CI:0.59590.9238,P=0.0189); sensitiv-ity and specificity were 89.0% and 61.3%6, respectively figure 3). MM patients were divided into two groups: RBC lifespan >61 d and RBC lifespan <61 d The ORR was 95.0% for 20 patients with RBC lifespan >61 d while the ORR was 60. 0% for 20 patients with RBC lifespan <61 d. The differences in ORR between these two groups was determined to be statistically significant (P=0.0080).
4. Discussion
The main underlying cause of anemia in MM patients had been assumed to be inadequate hb production due to bone destruction and marrow failure. However anemia can be aggravated by shortened RBC survival The role of rbc survival in hematologic tumors remains poorly understood owing to the lack of simple, rapid, noninvasive technologies to assess RBC lifespan. In the present study, we used a simplified instrument for the quantification of endogenous CO and estimation of RBC survival in MM patients for the first time.
Our results showed a mean RBC lifespan of 116+ 1, d for healthy subjects, which is consistent with the results of previous reports using traditional (114±20d) and co methods(122±23d)[22,23 Mean RBC lifespan for MM patients(63 t 23)was significantly lower than that for healthy controls. Sur-prisingly, RBC lifespan in non-anemic MM patients (78+ 21 d) was also shortened than healthy controls, suggesting decreasing RBC lifespan is a common henomenon in mm patients, regardless of anemia or not. The abnormal expression of inflammatory cytokines, including IL-1, IL-6, TNF-α, IFN-γ,are well known to play crucial roles in the pathogenesis and progression of MM [24, 25]. IL-1, IL-6, and TNF a released by cancer cells can activate immune cells reduce ervthrocyte lifespan, and increase ervthrocyte destruction by macrophages [26-28. Therefore, the inflammatory state of MM patients may involve in shortened erythrocyte survival. Of course, this spec alation needs to be further validated by detecting the level of inflammatory cytokines in MM patients. RBC lifespan of MM patients with anemia(52 18 d)was significant lower than those without anemia. This indicates that mild to moderate decreases in RBC sur-vival can be compensated by EPO-ependent increa-ses in RBC production. However, severely shortened RBC lifespans, like AIHA, may aggravate the reduction of Hb concentration and cause anemia.
Our study found that mm patients with anemia often suffer from renal impairment. The incidence of renal impairment was 30.4% in the 23 patients with anemia, while the incidence was only 5.9% in the 17 patients without anemia. There was a significant inverse correlation between RBC lifespan and serum creatinine level. Previous study showed that RBC life span was decreased in patients with renal failure [29] In addition, the RBC lifespan declined when blood from normal donors was transfused into uremic patients. However, blood from uremic patients trans fused into normal recipients resulted in a normal RBC survival. All these suggested that nephrotoxin con tribute to the shortening of RBC lifespan. Uremia may be a key factor that causes a loss of RBC surface lipids and interferes with intracellular glycolytic metabolis resulting in a shortened RBC lifespan [30,31]. Besides, relative deficiency of EPO levels may also par-ticipate in the anemia of MM patients with renal insuf ficiency. Capalbo et al reported that EPO levels were higher in hematologic neoplastic anemia patients than healthy s but the levels could not match the degree of decline in Hb [32]. EPO resistance induced by inflammatory cytokines could also aggra vate anemia[33].
The VCd protocol has precedent of being used as a first-line induction therapy for MM [34]. To study the effects of RBC lifespan on VCD chemotherapy, a cut-off value of 61 d was established using an ROC curve.The ORR of MM patients with RBC lifespan ≥ 61 d was significantly higher than those with RBC lifespan <61 d. There was a significant inverse correlation between RBC lifespan and clonal plasma cell percent-age, β2-MG level, and serum creatinine level, indicat-ing that RBC lifespan reflect the degree of tumor burden of MM patients indirectly. The shorter the RBC lifespan, the higher the degree of tumor burden in mm patients. Therefore RBC lifespan may be used as a factor to predict the chemotherapeutic effect. Fur-ther studies are warranted to determine the role of RBC lifespan with a greater sample size and longer fowell-up.
5 Conclusions
The current study provided a simple and accessible means with which to assess RBC lifespan in MM patients based on exhaled CO. RBC lifespan is decreased in MM patients, especially in anemia MM patients. A severely decreased RBC survival may play significant role in anemia of MM patients. The exact mechanisms of reduced RBC lifespan in MM are unknown, inflamma tory cytokines and renal toxins may play a pivotal. We believe this study will show the potential clinical value for the assessment of RBC survival in hematological patients.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (81700192).
Conflict of interest disclosure
The authors declare no conflicts of interest.
ORCID iDs
YanfangWang https:/orcid.org/0000-0002-4956-8209
FeiDong https:/orcid.org/0000-0002-2964-9562
References
[1] Kumar SK, Rajkumar V, Kyle A, van Duin M, Sonneveld P, Mateos M V, Gay F and Anderson K C 2017 Multiple myeloma Nat Rev. Dis. Priimers317046-55.
[2] Rajkumar S V 2016 Multiple Myeloma: 2016 updateon iagnosis, risk-stratification and management Am J.Henatology91719-34.
[3] Terpos Eet al 2015 European myeloma network guidelines for the management of multiple myeloma-related complication Haematologica 100 1251-66.
[4] Birgegard G 2008 Managing anemia in lymphoma and multiple myeloma Ther. Clin. Risk Manage 4527.
[5] Qian J, Jin J, Luo H, Wang L, Qian Wand Meng H2017 Analysis of clinical characteristics and prognostic factors of multiple myeloma: a retrospective single-center study of787 cases hematology 22 472-6.
[6] Caro ]J, Salas M, Ward A and Goss G 2001 Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review Cancer 912214-21
[7] Bouchnita A, Eymard N, Moyo T K, Koury M Jand Volpert V 2016 Bone marrow infiltration by multiple myeloma causes anemia by reversible disruption of erythropoiesis Am .J. Hematology 91371-8.
[8] Nairz M, Theurll, Wolf D and Weiss G 2016 Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia ofinflammation Wiener med Wochenschr 166 411-23.
[9] Ludwig H, Pohl G and Osterborg A 2004 Anemia in multiple loma Clin. Adv. Hematology Oncol. 2 233-41
[10] TucciM, Grinello D, Cafforio P, Silvestris F and Dammacco F 2002 Anemia in multiple myeloma: role of deregulated plasma cell apoptosis Leukemia Lymphoma 43 1527-3
[11] Berlin N I, Lawrence ]H and Lee HC 1951 The life span ofthe red blood cell in chronic leukemia and polycythemia Science 114385-7
12] Weiss G and Goodnough L T 2005 Anemia of chronic diseas En.J.Med.3521011-2313] Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni v and Maccio A 2018 Pathogenesis and treatment nechanism-based approach Front Physiol. 9 1294
[14] Ebaugh F G, Emrson CP and Ross F1953 The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination of red cell survival in vivo J Clin. Invest, 32 1260-76
[15] SuzukiT and Dale GL1987 Biotinylated erythrocytes: in vi urvival and in vitro recovery Blood 70791-5
[16] Franco RS2009 The measurement and importance of red cell survival Am .J. Hematology 84 109-14
[17] Strocchi A, Schwartz S, RR, Medina a and Levitt MD 1992 A simple carbon monoxide breath test to imate erythrocyte turnover J. Lab. Clin Med. 120 392-9
[18] Coburn 1970 Endogenous carbon monoxide production New Engl J Med. 282 207-9
[19] Coburn R The measurement of endogenous carbon noxide production Appl. Physiol. 112 1949-55
[20] Zhang H Det al 2018 Human erythrocyte lifespan measured b Levitt' s CO breath test with newly developedautomatic instrument J.Breath Res. 120306003-09
[21] Rajkumar S vetal 2014 International myeloma working group updated criteria for the diagnosis of multiple myeloma Lancet Oncol.15c538-48
[22] Dinant H ] and DeMaat C E M Erythropoiesis and mean red ell lifespan in normal subjects and in patients with the anaemia of active rheumatoid arthritis Br.J.Hematology 39 437-44
[23] Mitlyng B L, Singh JA, Furne jK, Ruddy J and Levitt M D 2006 Use of breath carbon monoxide measurements to assess erythrocyte survival in subjects with chronic diseases A. J Hematology 81 432-8
[24] Filella X, Blade J, Guillermo A L, Molina R, Rozman C and Ballesta A M 1996 Cytokines(IL-6,TNF-alpha,IL-1alpha) and soluble interleukin-2 receptor as serum tumor markers in multiple myeloma Cancer Detect Prev 2052-6
[25] Lee S J and Borrello I 2016 Role of the immune response in disease progression and therapy in multiple myeloma Cancer Treat Res 169 207-25
[26] Musolino C, Allegra A, Innao V, Allegra A G, Pioggia g and Gangemi S 2017 Inflammatory and anti-inflammatory equilibrium, proliferative and antiproliferative balance: the role of cytokines in multiple myeloma Mediators Inflammation 20171852517-30
[27] MeansRT1995 Pathogenesis of the anemia of chronic disease a cytokine-mediated anemia Stem Cells 13 32-7
[28] Salvarini C, Casali B, salvo D, Brunati C, MacchioniPL Massai g, Lasagni D, Rivasi P and Portioli l 1991 The role of interleukin 1, erythropoietin, and red cell bound immunoglobulins in the anaemia of rheumatoid arthritis clin Exp Rheumatol. 9241-6
[29] Ly J, Marticorena R and Donnelly $2004 Red blood cell survival in chronic renal failure Am J. Kidney dis. 44715-9
[30] Loge JP, Lange RD and Moore Cv1958 Characterization of the anemia associated with chronic renal insufficiency Am J Med.2441831] KuroyanagiT1961 Anemia associated with chronic renal failure, with special reference to kinetics oferythron nihon Ketsueki gakkai zasshi 24 156-75
[32] Capalbo S, Battista C, Delia M, Ciancio A, De Santis G Dargenio M, Diomede d and liso v 2002 Evaluation of tumor necrosis factor-alpha and erythropoietin serum levels in B-cell chroniclymphocytic leukemia patients with anemia Acta Hematology 108 84-9
[33] Cardia, Paoletti E, De Nicola L, Mazzaferro S, Russo R and Cozzolino m 2013 Renal anaemia and ePO hyporesponsiveness associated with vitamin d deficiency the potential role of inflammation Nephrol. Dialysis Transplant. 281672-9
34 Vigolo S, Zuckermann J, BittencourtRl Silla l and Pilger DA 2017 Comparison of cyclophosphamide-thalidomide dexamethasone to bortezomib-cyclophosphamide-dexameth asone as induction therapy for multiple myeloma patients in Brazil hematology oncol Stem Cell Ther. 10 135-42



