Human erythrocyte lifespan measured by Levitt's CO breath test with…
Human erythrocyte lifespan measured by Levitt's CO breath test with newly developed automatic instrument
Hou-De Zhang1,2, Yong-Jian Ma9,1, Qi-Fa Liu3, Tie-Zhen Ye4, Fan-Yi Meng3, Yi-Wen Zhou5, Guo-Pan Yu3, Jian-Ping Yang4, Hua Jiang4, Quan-Shi Wang6
Abstract
Existing standard techniques for erythrocyte (RBC) lifespan measurement, such as quantitation of labeling with isotopes or biotin, are cumbersome and time-consuming. Given that endogenous CO originates mainly from degraded RBCs, a team lead by Levitt developed a CO breath test to enable more efficient RBC lifespan estimation. The purpose of this study was to evaluate the reliability of Levitt's CO breath test method with our newly developed automatic instrument. RBC lifespan measurements conducted by Levitt's CO breath test method were conducted in 109 healthy subjects and 91 patients with chronic hemolytic anemia. In healthy subjects, the RBC lifespan was 126 ± 26 days, similar to values obtained with classical standard labeling methods. RBC lifespan did not differ significantly between males and females or between juveniles and adults, and did not correlate with age. To our knowledge, this datum represents an RBC lifespan average for the largest sample to date. In subjects with hemolytic anemia, RBC lifespan was 29 ± 14 days, which is significantly shorter than that of the healthy subjects (p = 0.001). Using 75 days as a cut-off, diagnostic accuracy for hemolytic anemia in the present study sample was 100%. In conclusion, the present results indicate that Levitt's CO breath test is an ideal method for human RBC lifespan measurement, and the newly developed automatic instrument is reliable and convenient for clinical practice.
1. Introduction
Erythrocyte (RBC) lifespan refers to the duration of time that RBCs survive in circulation after they are released from bone marrow. RBC survival characteristics are useful in both clinical and research settings. The commonly referenced normal human adult RBC lifespan of about 120 days was derived from the transfused allogeneic RBC survival time, which was determined by the differential agglutination technique introduced by Winifred Ashby [1–4] in 1919. There are three standard techniques that are used to measure autologous RBC lifespan, namely stable isotope 15N-glycine labeling, radioactive isotope 51Cr labeling, and biotin labeling.
For the 15N-glycine labeling technique of determining RBC lifespan, which was developed in a cohort study [5, 6], a specified dose of 15N-glycine, a nitrogenous precursor of the porphyrin rings in heme, is taken orally. Newborn RBCs produced in the bone marrow incorporate heme labeled with the administered 15N-glycine. The isotope concentration of heme in peripheral blood rises rapidly as the newly labeled RBCs are released, remains stable for a period of time while the labeled RBCs are in circulation, and then declines sharply thereafter as the labeled batch of RBCs die off. The amount of time between the rapid increase and rapid decrease phases is taken as an index of average RBC lifespan. For the 51Cr-labeling technique, which was developed in a population study [7–9], a small peripheral venous blood sample is taken, incubated with radioactive Na2 51CrO4, and then re-infused back into circulation.51Cr binds tightly but noncovalently to hemoglobin. Because circulating blood contains RBCs of a span of ages, re-infused 51Cr-labeled cells die over a period of time, rather than all at once. Hence, the rate at which 51C radioactivity disappears can be used to estimate RBC lifespan. Finally, in the biotin labeling technique, which is performed by a re-perfusion protocol similar to that used for 51Cr-labeling [10–12], RBCs are labeled with biotin as it binds covalently to RBC membrane proteins. Following secondary binding of RBC-bound biotin with fluorochrome-conjugated streptavidin, biotin labeled RBCs can be quantitated by flow cytometry. The biotin labeling test has the advantage of not involving radiation exposure, enabling it to be used in pregnant women and neonates.
The aforementioned methods have several major drawbacks. First, they require multiple venesections. Second, these multiple procedures must be conducted over a period of at least several weeks, and in some cases several months. Third, a hematological steady state must be maintained throughout the measurement period because any fluctuations may alter the detected lifespan. These drawbacks render all three of these labeling methods inconvenient for both animal research and clinical practice applications.
Endogenous CO originates mainly from the heme oxidation that occurs during the hemoglobin degradation that follows RBC rupture [13–15]. As early as 1966, Courn et al [16] reported that endogenous CO production rate was an accurate index of RBC survival, relative to a 51Cr-labeling method standard, in patients with hemolytic anemia. However, Courns method requires a complex rebreathing protocol and a measurement of total blood volume, which is impractical in clinical practice. As a result, use of elevated exhaled CO measures has been limited mainly to the qualitative assessment of hemolytic anemia in pediatric patients [17–19].
In 1992, Levitt's research team [20, 21] developed the first rapid CO breath test, which could be used to calculate RBC lifespan based on exhaled alveolar endogenous CO and blood hemoglobin concentration. Levitt's CO breath test has been reported to provide a quantitative assessment of RBC survival status [20–30]. Moreover, Levitt's test has been shown to be suitable for uses beyond steady state measurements; indeed, it remains the only available method with which to estimate short-term fluctuations in RBC destruction [31]. There is a simplified version of Levitt's CO breath test available, known as the modified Levitt's CO breath test [21]. However, gas sample measurements in the modified Levitt's CO breath test still require a number of sophisticated instruments that should be operated by skilled professionals. In addition, studies of the modified Levitt's CO breath test have been limited by small sample sizes [20–30].
With the long-term aim of facilitating implementation of a CO breath test for routine use in clinical practice, we developed a fully automated instrument based on the principle of Levitt's CO breath test that can be operated proficiently following limited training. Our newly developed instrument has been demonstrated to provide reliable measurements of low-concentration CO gas and RBC lifespan in small animal studies [32, 33]. The purpose of this study was to evaluate the reliability of Levitt's CO breath test method with our newly developed automatic instrument. Specifically, we tested (1) whether the mean normal RBC lifespan value obtained with the automatic instrument fit with the standard approximation of 120 days, and (2) whether this new instrument can be used to identify a shortened RBC lifespan in patients with hemolytic anemia.
2. Materials and methods
2.1. Study subjects
The first experiment was designed to assess whether the mean normal RBC lifespan value obtained with the newly developed instrument fit with the standard approximation of 120 days. A group of 104 nonsmoking healthy subjects, including 46 children of medical staff at Guangzhou Women and Children Medical Center and 58 adult personnels at Shenzhen University, were recruited. The subjects ranged in age from 7 years to 70 years old (mean ± standard deviation (SD), 21.5 ± 11.4 years). Thus, there were juvenile (range, 7–17 years; 10.6 ± 2.6 years) and adult (range, 18–70 years; 30.1 ± 7.6 years) subgroups within the healthy group. The healthy group included 56 females (53.8%), including 21 girls and 35 women. All subjects were free of a chronic disease history and abnormal physical examination signs. None had experienced an acute illness or taken medicine in the 8 weeks preceding or during the study. All 104 subjects had normal blood hemoglobin concentrations (range, 120–168 g l−1), hematocrit levels, mean RBC volumes, RBC distributions, reticulocyte counts, erythrocyte osmotic fragility test results, Coombs test results, hemoglobin electrophoresis results, and erythrocyte glucose-6-phosphate dehydrogenase activity levels. Additionally, all 104 subjects had normal results on clinical routine tests (blood, urine, and stool) as well as normal hepatic and renal function test results. Exclusion criteria were severe chronic cardiopulmonary diseases, acute diseases or emergent medical needs, pregnancy, breastfeeding, a blood transfusion within 3 weeks of the study, having participated in another clinical trial in the preceding 3 months, and active smoking, severe passive smoking or other similar air contamination exposure within 24 h before the test.
For the second experiment, designed to examine the capacity of the breath test with the instrument to detect an abnormally short RBC lifespan, a group of 91 nonsmoking patients with chronic hemolytic anemia, including 89 with thalassemia, 1 with hereditary spherocytosis, and 1 with autoimmune hemolytic anemia, were enrolled. The age (21.5 ± 11.4 years) and gender (male/female = 54/37) profile of the group was comparable to that of our healthy subjects group. Chronic hemolytic anemia was diagnosed based on each patients history, physical examination findings, and routine laboratory data. All 91 patients in the hemolytic anemia group had a blood hemoglobin concentration below 120 g l−1(range, 38.0–105.0 g l−1), elevated RBC destruction, and compensatory erythroid proliferation. Thalassemia was confirmed by hemoglobin electrophoresis and genetic analysis. Hereditary spherocytosis was confirmed based on the presence of spherical-shaped erythrocytes (≥10% RBCs) on a peripheral blood smear and a positive familial history, and autoimmune hemolytic anemia was confirmed by positive direct antiglobulin test results and normal titers of serum cold agglutinin. Exclusion criteria were the same as in the healthy group.
The study protocols (registration no. CHiCTR-DDD17011592) were approved by the Review Boards of Nanfan Medical University and Guangzhou Women and Childrens Medical Center. The experiments were carried out in accordance with the Declaration of Helsinki. Before participation in the study, written informed consent was obtained from each adult subject and from a parent of each juvenile subject.
2.2. Sampling
Alveolar air samples were collected in the morning (8.00–11.30) without a fasting requirement. Briefly, after a deep inspiration, each subject held his or her breath for 10 s, and then exhaled into a collection system through a mouthpiece (figure 1(A)). The collection system discards the first 300 ml of dead space gas and then directs subsequent alveolar air automatically into a foil collection bag. If needed, the procedure was repeated until the collected air sample reached the collection bags capacity of 1000 ml. The filled bag was detached and sealed. Atmospheric samples were collected just after breath sampling. Alveolar air and atmospheric samples were stored at room temperature and analyzed within 5 days. On the same day as alveolar air sampling, peripheral vein blood samples were collected for routine hemoglobin measurement.
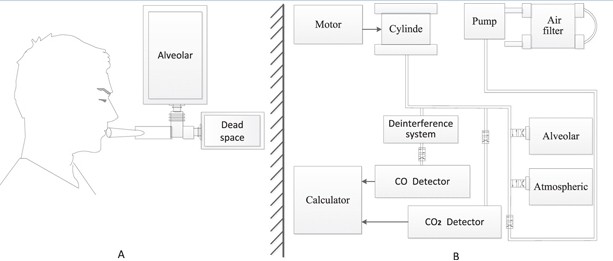
2.3. Instrument and measurement
Our newly developed automated instrument (ELS TESTER, Seekya Biotec Co. Ltd, Shenzhen, China) determines alveolar endogenous CO concentration by non-dispersive infrared spectroscopy with paired alveolar and air gas samples, and uses that measurement as a basis for determining RBC lifespan with Levitt's formula [20, 21, 32, 33]. The instrument design is illustrated in figure 1(B). The detection limit of the instrument for CO is 150 ppb, with an accuracy of ±50 ppb and precision within 50 ppb. The instrument is easy to operate. Simply put the instrument operator, connects paired air-alveolar gas samples to inlets, enters the subjects blood hemoglobin concentration data, and then presses the start button, triggering the instrument to complete a series of automated measurements encompassing the following steps. First, the quality of a collected alveolar sample is checked by measuring its CO2concentration, which is used as a dilution indicator of the alveolar sample. A small volume of alveolar air is pumped into the CO2 detection chamber and measured for digital voltage under infrared light (wavelength, 4.26 μm). The digital voltage is converted to CO2 concentration via a pre-imputed calibration curve obtained with standard CO2 gas samples (voltage/CO2 concentration curve). Only alveolar gas samples containing <5% CO2 are considered to be diluted. Second, to eliminate molecules that can interfere with CO infrared detection, the sample gas is propelled into a de-interference system with an absorbent mixture consisting mainly of soda asbestos. The absorbed interference molecules are mainly H2O and CO2. Third, a paired measurement technique is used to determine the CO concentration difference between alveolar and atmospheric air. The two de-interfered samples are pumped in series into the CO detection chamber and digital voltage is measured under infrared light (wavelength, 4.65μm). The digital voltage difference between the paired samples is derived by subtraction. That digital voltage difference is then converted to a CO concentration difference, which is recorded as the endogenous alveolar CO concentration via a pre-imputed calibration curve obtained with a series of paired standard CO gas samples of known concentrations (voltage difference/CO concentration difference curve). Fourth, any endogenous alveolar samples found to be diluted (i.e. having <5% CO2) are normalized to a 5% CO2 status. Finally, RBC lifespan is calculated by Levitt's formula (see below) based on the parameters of the pre-imputed hemoglobin concentration and corrected (if necessary) endogenous alveolar CO concentration. The instrument reports the following data for the subject: alveolar CO2, endogenous alveolar CO, and RBC lifespan.
2.4. Levitt's formula
Metabolic CO is produced predominantly by conversion of the α-methene carbon of the porphyrin ring to CO during the catabolism of heme to bilirubin; degradation of hemoglobin from dying RBCs represents the majority of heme turnover [15]. Consequently, the CO production rate reflects RBC turnover. Because CO is excreted entirely via the lungs, pulmonary gas samples can be used to measure CO production [15]. Based on these principles, Levitts team [20] developed a simple noninvasive CO breath test for RBC lifespan estimation in human subjects in 1992. The test was modified by the same team in 2003 [21]. The endogenous component of alveolar breath CO is determined by subtracting atmospheric PCO from alveolar Pco. RBC lifespan (in days) was calculated based on CO measurements from the following formula, which equates mean RBC lifespan to the total capacity of CO from hemoglobin divided by the CO quantity released per day.
where [Hb] is the hemoglobin concentration in g ml−1, Vb is total blood volume, 22 400 is the molar quantity of CO in milliliters, and 4 is the number of moles of CO bound to each mole of hemoglobin. In the denominator, endoPco is the alveolar CO concentration in ppm, Vt is the volume of resting alveolar ventilation, 0.7 is the approximate fraction of CO production derived from circulating hemoglobin turnover, 64 400 is the molecular weight of hemoglobin, and 1440 is the number of minutes in 1 day. Because blood volume and resting alveolar ventilation both tend to vary directly with alveolar surface area and have roughly similar magnitudes when blood volume is expressed in milliliters and ventilation is expressed in milliliters per minute, these two values cancel out in this equation. Therefore, the equation can be simplified to the following expression, which we refer to as Levitt's formula.
Equation (2)
2.5. Statistics
Data obtained from the normal subject group are expressed as means ± SDs and coefficients of variation were calculated by dividing SDs by their associated means. Data normality was determined by way of the moment method. RBC lifespan means were compared across gender and age groups with Students t-tests. Correlation coefficients were determined with the Spearman correlation test. The normal value range was considered to be 95% of the range of a dataset with a normal distribution.
For hemolytic anemia diagnosis confirmation, we used receiver operating characteristic (ROC) curves to establish an optimal discriminate value. The diagnosis accuracy rate was calculated as the number of correctly diagnosed cases divided by the total number of (normal and hemolytic) subjects.
3. Results
The mean alveolar sample CO2 concentration of 104 healthy subjects was 5.8 ± 0.6%, and that of 91 patients with hemolytic anemia was 6.1 ± 2.5% (t = 12.016, P > 0.05). Twenty samples (9.6%) contained <5.0% CO2, including 11(10.6%) from healthy subjects (range, 4.12%–4.94% CO2) and 9 (9.9%) from patients with hemolytic anemia (range 3.54%–4.94% CO2, P > 0.05), and were thus considered to be diluted. The endogenous alveolar CO concentration results were normalized to 5.0% CO2 status in these diluted samples. No sample contained more than 7.0% CO2. Sample quality was satisfactory.
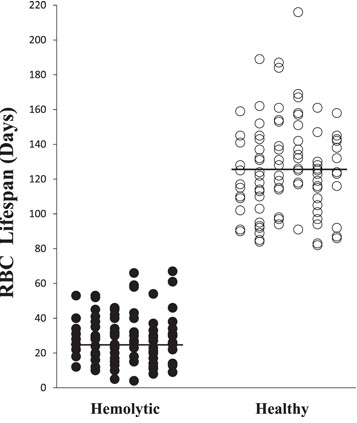
The mean endogenous alveolar CO concentration was 1.8 ± 0.5 ppm in healthy subjects and 5.9 ± 3.2 ppm inpatients with hemolytic anemia(t = 12.016, P < 0.0001). The RBC lifespan determined by Levitt's formula for each individual subject are shown in figure 2. Note that there is no overlap between the two groups datasets.
3.1. Normal RBC lifespan
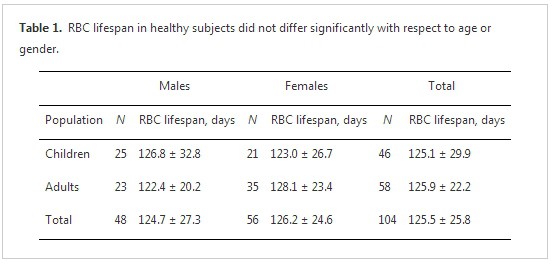
The mean RBC lifespan observed for the healthy volunteer group was 126 ± 26 days (range, 82–215 days; coefficient of variation = 0.21). Mean RBC lifespan did not differ significantly between children and adults, nor between males and females (table 1). RBC lifespan did not correlate with age (r = 0.05). The data were confirmed to have a normal distribution; 95% of the range (75–177 d) was within 1.96 SDs of the mean. There were no RBC lifespan data points less than 75 days, but four data points (3.8%) exceeded 177 days (i.e., 184 days, 187 days, 189 days, and 216 days).
3.2. Diagnostic performance for hemolytic anemia
The RBC lifespan of patients with hemolytic anemia was significantly shorter than that of the normal subjects (29 ± 14 days versus 126 ± 26 days, p = 0.001). ROC curve analysis showed that the optimal threshold of discrimination between the normal and hemolytic anemia groups was 75 days, a time interval that coincided with the lower limit of the 95% range of the normal group values. Using this cut-off value, the diagnostic accuracy for hemolytic anemia in our study sample was 100%.
4. Discussion
In this study, we used a newly developed instrument based on Levitt's CO breath test principles to measure RBC lifespan in 109 healthy subjects and 91 patients with chronic hemolytic anemia. In healthy subjects, the average RBC lifespan was 126 days with a 95% inclusion range of 75–177 days. The mean obtained for the healthy group was very close to values attained previously by classical standard methods (table 2). To our knowledge, this is the largest sample of RBC lifespan measurement data obtained in healthy subjects to be reported to date. We found that RBC lifespan of patients with hemolytic anemia was significantly shorter than that of the healthy subjects. Using a 75 day cut-off, we obtained a diagnostic accuracy for hemolytic anemia of 100% in our study sample. The results indicate that Levitt's CO breath test approach, as realized in our newly developed instrument, is reliable for the measurement of RBC lifespan in children and adults.
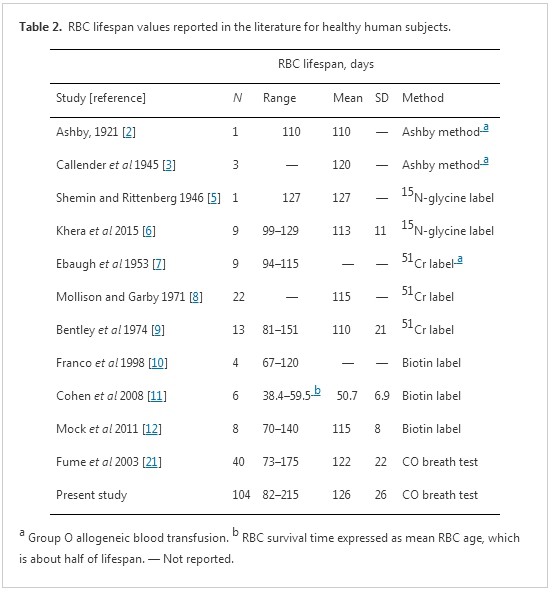
A variety of methods have been utilized to measure human RBC lifespan over the last near century. The first accurate method for determining RBC lifespan took advantage of emerging knowledge of the ABO blood-type system introduced by Winifred Ashby in 1919 [1, 2]. At that time, it was widely believed that the nucleus-free RBC was fragile with a limited, than the lifespan of perhaps 2–3 weeks [4, 34]. Ashby used anti-A and anti-B antiserum to measure the lifespan of type O RBCs that had been transfused into anemic type A or type B recipients and observed much longer RBC survival times than expected. Indeed, there was one reported case of a man who continued to show evidence of some transfused RBCs 110 days after receiving a blood transfusion for hemorrhage while otherwise being in good health [4]. Although controversial for many years, Ashby's observations were ultimately fully vindicated. In 1945, using Ashby's differential agglutination method, Callender et al [3] concluded from a mathematical analysis of data obtained from a group of three Rh-positive male medical students who volunteered to undergo blood withdrawal and equal-volume transfused blood replacement that RBCs live for approximately 120 days. Since the development of highly specific isotope tracer techniques, such as 15N-glycine and 51Cr labeling tests, this lifespan has become widely accepted as the normal mean human RBC lifespan. Notwithstanding, the early erythrocyte survival experiments conducted with the Ashby method continue to be regarded as a milestone of erythrocyte physiology research [34]. Indeed, as shown in table2, the normal mean human RBC lifespan obtained with a modified Levitt's CO breath test was 122 day in the original study [21] and 126 days in the present study. Values obtained with breath test studies are consistent with prior findings obtained with Ashby's differential agglutination method and test findings obtained with complex standard labeling methods.
The normal range of human RBC lifespan derived in the present study (75–177 days) is comparable to ranges obtained based on standard labeling methods (70–140 days) [10–12]. As shown in table 2, however, the actual reported range of RBC lifespan in healthy subjects with Levitt's CO breath test (73–215 days) is larger than that based on standard labeling methods (67–151 days). Three explanations may be application to this phenomenon. Firstly, it may be related to the principles underlying the different measurement methods. CO measured in breath tests originates from ineffective erythropoiesis in the bone marrow and destruction of peripheral circulating RBCs, such that the RBC lifespan is an average of these two components of RBC destruction [16, 35, 36]. In 15N-glycine or 14C-glycine labeling studies, RBCs are labeled during their production in bone marrow and can differentiate into short-lived and long-lived cell populations. The presence of very short-lived cells or ineffective erythropoiesis in bone marrow will reveal a distinct early decrease in blood label concentration prior to the plateau phase, and these very short-lived cells are not included in the calculation of RBC lifespan with these methods [6, 37]. Meanwhile, population labeling studies, such as with 51Cr or biotin, reflect RBCs of all ages in peripheral circulation, without being influenced by erythropoiesis in the bone marrow. Secondly, the range difference may be related to sample size. The use of much larger population samples in breath test studies than in standard labeling methods studies may have revealed a greater span of inter-individual variation. It is difficult to carry out large-sample studies in healthy volunteers with standard labeling methods because the process is cumbersome and time-consuming, requiring weeks or even months to carry out (table 2). In fact, the largest sample size of healthy volunteers in the reported labeling studies was only 22. Most others had no more than 10 participants. The recommended normal range of 70–140 days was derived from a biotin labeling study with only 8 healthy adults [10–12]. Thirdly, errors and deviations may arise during breath sample collection, storage, and measurement. In the present study, there were no RBC lifespan data points less than 75 days, but four data points (3.8%) exceeded 177 days (i.e., 184 days, 187 days, 189 days, and 216 days). According to Levitt's formula, the lower the CO concentration that is measured, the longer the longer RBC lifespan will be. As a result, extremely long RBC lifespan data may be artefacts of sample gas leakage. Further studies are needed to resolve this question.
Gender-and age-related variations in peripheral RBC count and hemoglobin concentration have been observed, with higher values being observed in males and in infants. However, gender- and age-related physiological variations in RBC indices, including mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration, have not been documented [38]. However, these factors have not been analyzed in RBC lifespan measurement, perhaps due to limited study cohort sizes. We did not observe evidence of RBC lifespan being related to gender or age in our study. Studies that employed standard methods have shown a shorter RBC lifespan in infants (including neonates) than in adults, even with adult-to-infant allogenic transfusions [39–42]. This phenomenon may reflect a greater need for RBC renewal in infants due to their relatively high metabolic rate. However, further work is needed to clarify how RBC lifespan evolves developmentally. The minimum age of enrolled subjects in our study was 7 years old; infants and toddlers were not included due to sampling challenges.
A shortened RBC lifespan is a fundamental characteristic of hemolytic anemia. As shown in figure 1, there was no overlap and very significant dissociation between our healthy and anemic groups. This observation is consistent with the findings of previous reports [16, 20, 21]. Using the 75 day cut-off value indicated by our ROC curve analysis, we were able to obtain 100% diagnostic accuracy for hemolytic anemia. Such excellent results confirm that RBC lifespan is indeed the gold standard for diagnosis of hemolysis. Additionally, they suggest strongly that simple, rapid, and reliable Levitt's CO breath tests are sufficiently convenient for regular clinical practice.
Beyond the theoretical principle, a key factor in determining Levitt's CO breath test accuracy for RBC lifespan assessment is the precise measurement of endogenous alveolar CO at a very low concentration. Although infrared spectroscopy for CO measurement is very sensitive, it is vulnerable to interference from H2O and CO2 [43]. In addition to eliminating H2O and CO2 from the sample, we adopted the following innovative measures for our instrument to improve measurement accuracy: (1) automated flushing of the measuring chamber with a cleaning gas to eliminate contamination before each sample is inputted; (2) paired measurement of air-alveolar gas samples to eliminate counting errors caused by direct-current level drift and background noise; (3) monitoring and correction of alveolar gas dilution during sample collection based on CO2 concentration measurements for the alveolar sample. Moreover, the integrated automation design makes the instrument very easy for nonprofessionals to operate. Together with previous studies in animals [32, 33], the present findings demonstrate that the newly developed instrument tested herein has achieved its design goals.
The main limitation of this study was a lack of standard methods (e.g., 15N-glycine, 51Cr or biotin labeling) for within-subject comparisons. However, the mean normal RBC lifespan obtained with our breath test in healthy subjects is consistent with whose reported by standard methods, and patients with chronic hemolytic anemia were shown to have a significantly shorter RBC lifespan without any overlap with the normal subject range. Hence, these results indicate strongly that the present CO breath test methodology is accurate and that our instrument is reliable. Another limitation of this study is that very young children were not enrolled due to breath sampling difficulty. Finally, accurate determination of blood hemoglobin concentration, one of the parameters for RBC lifespan calculation, requires a separate invasive blood test. In the future, it would be ideal to develop a new generation instrument that can detect both alveolar CO and blood hemoglobin non-invasively.
5. Conclusions
Human RBC lifespan measured by Levitt's CO breath test with our newly developed automatic instrument is consistent with reported values obtained with more complex and time-consuming methods. The test provided clear and reliable discrimination between normal-range RBC lifespans in healthy subjects and reduced RBC lifespans in patients with hemolytic anemia. Although RBC lifespan is of known diagnostic significance for hemolytic anemia, RBC lifespan measurement is seldom used in routine clinical practice due to classical methods being cumbersome and time-consuming. A simple, rapid, and accurate CO breath test methodology based on Levitt's CO analysis principle, such as that examined in this study, can enable RBC measurements to be taken with ease in clinical settings.
Acknowledgments
The authors thank Hong Li, Sui-Song Zhu, Ze-Lin Liu and Jia-Liang Huang for their contribution to these studies.
1 Ashby W 1919 The determination of the length of life of transfused blood corpuscles in man J. Exp. Med. 29 267–81
2 Ashby W 1921 Study of transfused blood I the periodicity in eliminative activity shown by the organism J. Exp. Med. 34 127–46
3 Callender S T, Powell E O and Witts L J 1945 The lifespan of the red cell in man J. Path. Bact. 57 129–39
4 Ashby W 1948 The span of life of the red blood cell. A resume Blood 3 486–500 (PMID: 18914277)
5 Shemin D and Rittenberg D 1946 The lifespan of the human red blood cell J. Biol. Chem. 166 627–36 (PMID: 20276177)
6 Khera P K et al. 2015 Use of an oral stable isotope label to confirm variation in red blood cell mean age that influences HbA1c interpretation Am. J. Hematol. 90 50–5
7 Ebaugh F G Jr, Emrson C P and Ross J F 1953 The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo J. Clin. Invest. 32 1260–76
8 Mollison P L and Garby L 1971 Deduction of mean red-cell life-span from 51Cr survival curves Br. J. Haematol. 20 527–36
9 Bentley S A, Glass H I, Lewis S M and Szur L 1974 Elution correction in 51Cr red cell survival studies Br. J. Haematol. 26 179–84
10 Franco R S, Lohmann J, Silberstein E B, Mayfield-Pratt G, Palascak M, Nemeth T A, Joiner C H, Weiner M and Rucknagel D L 1998 Time-dependent changes in the density and hemoglobin F content of biotinlabeled sickle cells J. Clin.
11 Cohen R M, Franco R S, Khera P K, Smith E P, Lindsell C J, Ciraolo P J, Palascak M B and Joiner C H 2008 Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c Blood 112 4284–91
12 Mock D M, Matthews N I, Zhu S, Strauss R G, Schmidt R L, Nalbant D, Cress G A and Widness J A 2011 Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities Transfusion 51 1047–57
13 Coburn R F 1970 Endogenous carbon monoxide production New Engl. J. Med. 282 207–9
14 Coburn R F 2012 The measurement of endogenous carbon monoxide production J. Appl. Physiol. 112 1949–55
15 Vreman H J, Wong R J and Dir S 2000 Carbon monoxide in breath, blood, and other tissues Carbon Monoxide Toxicity ed D G Penney (Boca Raton, FL: CRC Press) pp 19–60 ch 2
16 Coburn R F, Williams W J and Kahn S B 1966 Endogenous carbon monoxide production in patients with hemolytic anemia J. Clin. Invest. 45 460–8
17 American Academy of Pediatrics Subcommittee on Hyperbilirubinemia 2004 Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation Pediatrics 114 297–316
18 James E B, Vreman H J, Wong R J, Stevenson D K, Vichinsky E, Schumacher L, Hall J Y, Simon J, Golden D W and Harmatz P 2010 Elevated exhaled carbon monoxide concentration in hemoglobinopathies and its relation to red blood cell
19 Lal A, Patterson L, Goldrich A and Marsh A 2015 Point-of-care end-tidal carbon monoxide reflects severity of hemolysis in sickle cell anemia Pediatr. Blood Cancer 62 912–4
20 Strocchi A, Schwartz S, Ellefson M, Engel R R, Medina A and Levitt M D 1992 A simple carbon monoxide breath test to estimate erythrocyte turnover J. Lab. Clin. Med. 120 392–9 (PMID: 1517686)
21 Fume J K, Springfield J R, Ho S B and Levitt M D 2003 Simplification of the end-alveolar carbon monoxide technique to assess erythrocyte survival J. Lab. Clin. Med. 142 52–7
22 Virtue M A, Fume J K, Nuttall F Q and Levitt M D 2004 Relationship between GHb concentration and erythrocyte survival determined from breath carbon monoxide concentration Diabetes Care 27 931–5
23 Nuttall F Q, Gannon M C, Swaim W R and Adams M J 2004 Stability over time of glycohemoglobin, glucose, and red blood cell survival in hematologically stable people without diabetes Metabolism 53 1399–404
24 Shima K, Chujo K, Yamada M, Komatsu M, Noma Y and Mizuguchi T 2012 Lower value of glycated haemoglobin relative to glycaemic control in diabetic patients with end-stage renal disease not on haemodialysis Ann. Clin. Biochem. 49 68–74
25 Virtue M A, Furne J K, Ho S B and Levitt M D 2004 Use of alveolar carbon monoxide to measure the effect of ribavirin on red blood cell survival Am. J. Hematol. 76 107–13
26 Mitlyng B L et al. 2006 Use of breath carbon monoxide to measure the influence of prosthetic heart valves on erythrocyte survival Am. J. Cardiol. 97 1374–6
27 Mitlyng B L, Singh J A, Fume J K and Levitt M D 2006 Use of breath carbon monoxide measurements to assess erythrocyte survival in subjects with chronic diseases Am. J. Hematol. 81 432–8
28 Krishnan S M and Dixit N M 2009 Estimation of red blood cell lifespan from alveolar carbon monoxide measurements Transl. Res. 154 15–7
29 Medina A, Ellis C and Levitt M D 1994 Use of alveolar carbon monoxide measurements to assess red blood cell survival in hemodialysis patients Am. J. Hemtol. 46 91–4
30 Sato Y, Mizuguchi T, Shigenaga S, Yoshikawa E, Chujio K, Minakuchi J and Kawashima S 2012 Shortened red blood cell lifespan is related to the dose of erythropoiesis-stimulating agents requirement in patients on hemodialysis Ther. Apher. Dial. 16 522–8
31 Franco R S 2009 The measurement and importance of red cell survival Am. J. Hematol. 84 109–14
32 Ma Y J et al. 2016 A modified carbon monoxide breath testfor measuring erythrocyte lifespan in small animals Biomed. Res. Int. 2016 7173156
33 Ma Y J, Zhang H D, Wu C H, Zhu G L, Ji Y Q, Huang J L, Du L T, Cao P, Zang D Y and Ji K M 2016 Rapid CO breath test screening of drugs for protective effects on ribavirin-induced hemolysis in a rabbit model: a pilot study J. Breath Res. 10 036010
34 Fairbanks V F 1975 In memoriam: Winifred M Ashby 1879–1975 Blood 46 977–8 (PMID: 1106797)
35 White P, Coburn R F, Williams W J, Goldwein M I, Rother M L and Shafer B C 1967 Carbon monoxide production associated with ineffective erythropoiesis J. Clin. Invest. 46 1986–98
36 Sannolo N, Farina V and Fiorillo A 1992 Abnormal endogenous carbon monoxide production in children with ineffective erythropoiesis Ann. Clin. Biochem. 29 397–9
37 Berlin N I, Lawrence J H and Lee H C 1951 The lifespan of the red blood cell in chronic leukemia and polycythemia Science 114 385–7
38 Fischbach F T and Dunning M B 2009 Manual of Laboratory and Diagnostic Test 8th edn (Philadephia, PA: Lippincott Williams and Wilkins) pp 56–62
39 Pearson H A 1967 Life-span of the fetal red blood cell J. Pediatr. 70 166–71
40 Fomon S J, Serfass R E, Nelson S E, Rogers R R and Frantz J A 2000 Time course of and effect of dietary iron level on iron incorporation into erythrocytes by infants J. Nutrition 130 541–5
41 Strauss R G, Mock D M, Widness J A, Johnson K, Cress G and Schmidt R L 2004 Posttransfusion 24 h recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants Transfusion 44 871–6
42 Widness J A, Kuruvilla D J, Mock D M, Matthews N I, Nalbant D, Cress G A, Schmidt R L, Strauss R G, Zimmerman M B and Veng-Pedersen P 2015 Autologous infant and allogeneic adult red cells demonstrate similar concurrent post-transfusion survival in very low birth weight neonates J. Pediatr. 167 1001–6
43 Dinh T V, Choi I Y, Son Y S and Kim J C 2016 A review on non-dispersive infrared gas sensors: improvement of sensor detection limit and interference correction Sensors Actuators B 231 529–38



