Use of breath Carbon monoxide to measure the influence of prosthetic…
Use of breath Carbon monoxide to measure the influence of prosthetic heart Valves on Erythrocyte Survival
Benjamin L. Mitlyng, MD, Yellapragada Chandrashekhar, MD, Julie k. Furne, BS and michael D. Levitt. MD*
First-generation prosthetic heart valves commonly caused sufficient red blood cell (RBC injury to induce hemolytic anemia. Although multiple studies have shown that new-generation valves are not associated with anemia, the extent to which these valves are injurious to RBCs is not known because RBC survival not has not been measured in these subjects Using a technique that uses breath carbon monoxide(co to quantify RBC turnover, this study measured RBC life span in 38 subjects with normally functioning, new-generation valves. Erythrocyte survival averaged 98.8+
23 and 103+ 15 days, respectively, in 20 subjects with mechanical valves and 18 subjects with bioprosthetic valves(p >0.05). However, these life spans were signifi-cantly (p <0.01)less than those of healthy subjects(122 23 days)and a group of elderly subjects with osteoarthritis (128+ 26 days). The mean hemoglobin concen trations of the 2 groups of valve patients were within normal limits. In conclusion, new-generation heart valves commonly are associated with a small degree of hemo-lysis that is compensated for by increased RBC production. o 2006 Elsevier Inc. All rights reserved (Am J Cardiol 2006: 97: 1374-1376)
Multiple studies have shown that currently used prosthetic heart valves are not associated with anemia, hyperbiliru binemia, and reticulocytosis, findings commonly observed with the severe hemolysis associated with first-generation valves. -4 However, several reports found a high prevalence of reduced serum haptoglobin concentration. 1. 2 a qualitative but sensitive indicator of intra- and extravascular hemolysis Thus, it seems likely that these valves may commonly induce subclinical hemolysis
Quantitative assessment of the injurious effect of prosthetic valves on erythrocytes requires the measurement of red blood cell(RBC) life span. A studys in which RBCs were pulse labeled at the time of their production in the marrow showed that the circulating concentration of labeled RBCs remained roughly constant for about 90 days. The labeled cells then disappeared over the ensuing 90 to 140 days. Thus, normally, there is minimal random removal of RBCS, but rather each cell has a relatively defined lifetime The RBC life span of the few subjects studied with this pulse-labeling technique averaged about 120 days, a value that has taken on the aura of a universal constant. In reality, subsequent studies have shown appreciable individual variability in the RBC life spans of healthy subjects. For example, techniques based on measurements of bilirubin production, 6 iron-59 kinetics,7 DF32P-labeled cells,8 and breath carbon monoxide (CO) excretion,9 respectively, found mean I SD values for RBC survival of104±14.103±23.124±7.and 122 23 days. The only technique commonly used to assess RBC life span in clinical practice has been the mea-surement of the disappearance of circulating RBCs labeled with chromium-51. Disadvantages of this technique include its(1) complexity (venesections over a 4- to 6-week period are required for a single life span measurement); (2) proviion of only semiquantitative results because of the variable elution of chromium-51 from RBCs; and (3) tendency to overestimate mean RBC survival because fragile cells are rapidly removed from the blood and therefore, are not circulating at the time blood is removed for labeling. 9 Al though used to measure the turnover of RBCs with first generation valves, this method has not been used in subjects with recently developed valves.
In the present study, we measured RBC survival using a simple, rapid, and noninvasive technique that uses the mea-surement of breath co concentration to estimate RBC sur vival. 10,11 The rationale of this technique is that all co generated in the body is derived from the a-methene carbon of heme which is stoichiometrically released as co when heme is converted to bilirubin. 2 Hemoglobin catabolism represents most heme turnover. Because all Co produced in the body is excreted in expired air, the measurement of CO concentration in a single expired air collection(corrected for atmospheric co)serves as a quantitative indicator of RBC life span. 9,10 Evidence of the validity of this technique includes the demonstration of normal(approximately 120-day) RBC life spans in healthy subjects and reduced spans in subjects with hemolysis documented by measurements of the disappearance of chromium- 51-labeled RBCs or fecal life bile pigment excretion. 9 The coefficient of variation of triplicate survival measurements obtained within a 5-minute period in 7 healthy controls averaged 3.0%. This technique is not applicable to smokers or subjects with badly impaired pulmonary function.
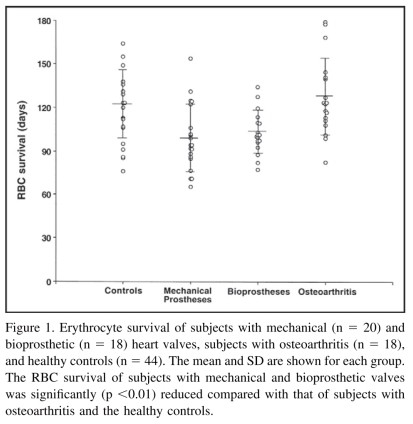
Erythrocyte survival was determined in 38 nonsmoking outpatients with new-generation prosthetic valves: 20(mean age 67+9) had mechanical valves(18 St Jude Mechanical St. Jude medical. Inc. St. Paul. Minnesota: 2 Medtron Hall, Medtronic, Inc, Minneapolis, Minnesota), and 18 (mean age 80+ 5 years) had bioprosthetic valves(11 Medtronic Mosaic, 7 Medtronic Hancock ) The valves were located in the aortic (n =30), mitral (n=5), or aortic and mitral(n=3) positions. None of the subjects had clinical or echocardiographic evidence of valve dysfunction, and none had clinical evidence of hepatic, renal, symptomatic pulmo-nary disease, or heart failure. Subjects were not taking drugs known to induce cytochrome activity(barbiturates, ri fampin, or dilantin). Control groups consisted of 44 previ-ously studied healthy controls10 and 18 outpatients(mean age 73+ 10 years)with osteoarthritis who served as an elderly group who did not have conditions associated with reduced RBC survival. The Co technique used was identi cal to that described in previous publications 10,11 The stud was approved by the institutional review board of the min neapolis veterans Affairs Medical Center and carried out according to the declaration of helsinki. Informed consent was obtained from all subjects.
The mean RBC survival for subjects with mechanical (99+23 days) and bioprosthetic (103+ 15 days) valves was not significantly different. However, the 2 values were signifi-cantly reduced (p <0.o1)relative to those of healthy subjects (122 23 days) and patients with osteoarthritis(127+ 25 days) (see Figure 1). Although decreased RBC survival with new-generation valves has been attributed to valv tion or paravalvular leaks, our patients had no com plications, and we conclude that a modest (mean about 20%) shortening of RBC survival occurs even with nor-mally functioning valves.
The mean hemoglobin concentration of our subjects with mechanical valves (14.1 1.2 g/dI) was in the normal range, whereas the hemoglobin of those with prosthetic valves(13. 1 g/dl) was within normal limits for elderly subjects(mean age 80 years). 3 Thus, increased erythrocyte output compensated for the decrease in RBC life span. This situation contrasts with that of subjects with anemia due to chronic disease, in whom we found that a roughly similar 25% reduction in RBC life span was associated with appre ciable anemia due to the failure of the marrow to enhance erythrocyte output(unpublished observation).
Seven of the 38 subjects with valves had low-grade anemia(hemoglobin <12 g/dl). However, there was no significant correlation (r=0.11,p >0.05) between RBC life span and hemoglobin concentration, and the RBC life spans(97+ 12 days)of the 7 anemic subjects were similar to those of the entire group of subjects with valves. Thus, the failure of the bone marrow to respond with increased output of erythrocytes(rather than increased RBC turnover) was the predominant factor accounting for the reduced he moglobin concentrations.
The mean reticulocyte percentages of subjects with mechan ical (1. 46 058%) and bioprosthetic (1.29 +0.48%0) valves fell well within the normal range(mean 1.1 %, range 0.5% to 2. 1%)despite the assumed increase in RBC output in many of these patients. This apparently contradictory finding is explained by the wide variation in reticulocyte percentages observed in healthy subjects(0.5% to 2.1%). The mean reticulocyte percentage of our subjects with valves(1.4+0.5%o)was roughly 30%0 greater than the normal mean of l1%. an increase consistent with the re duction in RBC life span observed in these subjects. How-ever, 36 of the 38 subjects had normal reticulocyte counts, and 2 had minimally elevated values. Thus, as observed previously, reticulocytosis is an insensitive indicator of low-rade hemolysis. 14
1. Okumiya T, Ishikawa- Nishi M, Doi T, Kamioka M, Takeuchi H, Doi Y, Sugiura T Evaluation of intravascular hemolysis with erythrocyte creatine in patients with cardiac valve prostheses. Chest 2004; 1252115-2120.
2. Suedkamp M, Lercher AJ, Mueller-Riemenschneider F, Larose K, Tossios P, Mehlhorn U. Hemolysis parameters of St Jude Medical Hemodynamic Plus@ and Regent( valves in aortic position. Int J Car-diol2004:95:89-93.
3. Erdil N. Cetin L, Ates s. Demirkilic U. Sener E. Tatar H. Cakir B Midterm experience with the Sorin Bicarbon heart valve prosthesis for rheumatic disease. J Cardiovasc Surg(Torino) 2003: 44: 597-603.
4. Aagaard J, Tingleff J. Fifteen years clinical experience with the Carbo-Medics prosthetic heart valve. J Heart Valve Dis 2005: 14: 82-88.
5. Shemin D, Rittenberg D. The life span of the human red blood cell.I Biol chen1946:166:627-636.
6. Berk pd, bloomer jr. Howe rb. blaschke tf. berlin ni. bilirubin production as a measure of red cell life span. J Lab Clin Med 1972;79:364-378.
7. Cavill I, Ricketts C, Napier JAF, Jacobs A, Ferrokinetics and eryth-ropoiesis in man: red-cell production and destruction in normal and anaemic subjects, BrJ Haematol 1977: 35: 33-40.
8. Eernisse JG, Van Rood JJ, Erythrocyte survival-time determinations with the aid of DF32P, BrJ Haematol 1961: 7: 382-404.
9. Strocchi A, Schwartz S. Ellefson M. Engel RR, Medina A. Levitt MD A simple carbon monoxide breath test to quantitate erythrocyte turn over. Lab Clin Med 1992: 120: 392-399.
10. Furne J, Springfield J, Ho SB, Levitt MD. Simplification of the end-alveolar carbon monoxide technique to assess erythrocyte survival J Lab Clin Med 2003: 142: 52-57.
11. Virtue MA, Furne JK, Nuttall FQ, Levitt MD, Relationship between Ghb concentration and erythrocyte survival determined from breath carbon monoxide concentration Diabetes Care 2004: 27: 931-935.
12. Landaw SA. Callahan EW, Schmid RE Catabolism of heme in-vivo Clin Invest 1970: 49: 914-925.
13. Smith DL. Anemia in the elderly. Am Fam Physician 2000: 62: 1565-1572.
14. Virtue MA. Furne JK. Ho SB. Levitt MD. Use of alveolar carbon monoxide to measure the effect of ribavirin on red blood cell survival Am J Hematol2004;76:107-113.



