Use of Alveolar Carbon Monoxide Measurements to Assess Red Blood Cell…
Use of Alveolar Carbon Monoxide Measurements to Assess Red Blood Cell Survival in Hemodialysis Patients
Angela Medina,Carol D.Levitt
Research Service,Veterans Affairs Medical Center(A.M.,C.E.,M.D.L.), and Department of Medicline (A.M.,M.D.L.),University of Minnesota School of Medicline, Minneapolis, Minnesota
We recently described a simple technique for measuring RBC survival based on measurements of the concentration of carbon monoxide(CO) in alveolar air,corrected for environmental CO using a device that equilibrates with atmospheric CO at the same rate as does the patient. The purpose of the present report is to demonstrate the clinical utility of this method via measurements of RBC turnover in hemodialysis patients. Prlor to dialysis, the mean RBC survival of 9 chronic dialysis patients was 70 ± 9 days, about 50% shorter than that of 32 healthy subjects. During the dialysis, the endogenous Pco increased by 14% this same time perlod. Thus. this technique demonstrated that the hemodialysis procedure resulted in about a 20% increase in RBC destruction. This increased RBC destruction has not been detectable with prevlous methodologies (including conventional measuretional techniques. We conclude that the simple, non-invasive measurement of endogenous Pco provides the most accurate available means of assessing the influence of a varietv of acute manioulations on RBC survival.
INTRODUCTION
Study of red blood cell (RBC) survival in health and disease has been hampered by the lack of a simple, reliable means of quantitating RBC turnover. The "goldstandard" for measurement of RBC life-span is cohort studies [1,2] which follow the disappearance of cells that have incorporated a labeled precursor such as [14C] glycine, technical complexity, and need to follow the patient through the senescence of the red cells (140 days in normals) severely limits the application of the method The only technique used with any frequency in the clinical setting involves the random-labeling method which follows the disappearance of 51Cr-labeled RBCs. This technique provides only semiquantitative data due to the elution of the isotope from the cells [3]. More important, this methodology is inconvenient since it requires multiple venesections over a several week period to obtain a single RBC survival measurement and, thus,cannot be used to study acute fluctuations in RBC turnover.
The only technique capable of quantitating rapid changes in RBC survival employs measurements of endogenous carbon monoxide (CO) production (Vco) to assess heme destruction[4,5]. The conversion of heme to bilirubin stoichiometrically releases CO, which is quantitatively excreted on the breath [4,6]. Thus, measurements of expired CO can be used to assess acute and chronic alterations in RBC turnover. However, the conventional method of measuring Vco requires that the patient rebreathe into a closed system for 3 or more hr [4-6] and , thus, requires a good deal of patient cooperation and, in addition, is too complex and time consuming for most applications.
Recently, we described a simple and rapid means of estimating Vco using measurements of end-alveolar Pco corrected for ambient CO exposure [7]. The purpose of the present report is to demonstrate the sensitivity and clinical utility of this technique via studies of RBC survival in hemodialysis Patients.
METHODS
Subjects
Hemodialysis patients. Nine subjects who were receiving regular hemodialysis were studied. None of the patients were smokers and none had received blood trans fusions during the preceding 2 weeks. End alveolar breath samples were obtained immediately before (ap proximately 12:30 PM)and at the end of the dialysis run which averaged about 4 hr in length. Dialysis was per-formed with Cobe 2RX dialyzers with cellulose acetate membranes. Dialysate was sequentially passed through a softener, carbon filters, reverse osmosis apparatus, and deionizer, and then subjected to UV light. The solute concentration of the dialysate usually consisted of Na 140 meq/l, CI: 110 meal, HCO3: 25-35 meq/l, Ca: 5 meq/l, Mg: I meq/l, K; variable, and glucose: 200 mg/dI The blood and dialysate flow rates were 300 ml/min and 550 ml/min, respectively. Vascular access was via two 15 gauge needles. The apparatus was primed with iso tonic saline, of which about 250 ml entered the circula tion of the patient. The estimated total blood loss during hemodialysis was less than 5 ml.
Healthy controls. Alveolar breath samples for co analysis were also obtained at 12: 30 PM and 4 30 PM in eight healthy laboratory technicians and secretaries who were carrying on their usual, relatively sedentary activI-ties.
CO Measurements
The technique employed to measure breath CO was identical to that described and validated in a previous publication [7]. End alveolar breath samples were col lected with a commercial device that automatically dis-cards the first 500 ml of expired air, and then collects the subsequent exhalation in a foil bag. To ensure that alvey-lar and blood Pco were in equilibrium, the subject held his breath for 20 sec prior to exhalation into the device Breath Pco was corrected for CO of environmental origin using air-filled syringes with a membrane-covered aper ture that allowed the air in the syringe to equilibrate with atmospheric Pco at approximately the same rate as did the patient. These"equilibrators"were maintained in the same environment as the patient for at least 8 hr prior to obtaining the breath sample. At each measurement time duplicate or triplicate breath samples were obtained along with an equilibrator sample.
Analysis. The Co concentration of end alveolar breath samples and the equilibrator were determined by gas hromatography using a gas sampling valve, a column (3 foot x 1/8 inch) packed with molecular sieve, oven tem-perature of 100C, argon as the carrier gas (40 ml/min)
and a reduction detector (Trace analytical).
Calculations. The endogenous Pco of a subject was determined frorm the difference between the end alveolar Pco and equilibrator Pco As described previously [7], RBC survival in days was calculated from endogenous Pco(ppm)and the hemoglobin concentration(g/ml)of a blood sample obtained prior to the dialysis run as follows:
RBC survival =(1,380 ml day per g)
(HGb]/Endogenous Pco).
The hemoglobin concentration of the pre-dialysis sample was used to both life-span measurements to avoid artifac tual changes in calculated survival resulting from changes in hemoglobin concentration secondary to fluid shifts during hemodialysis.
RESULTS
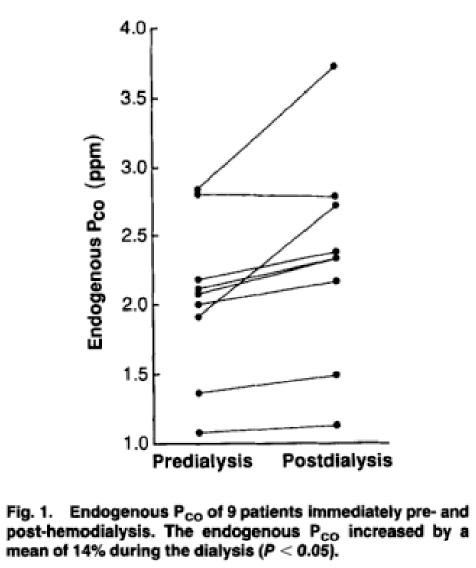
The endogenous Pco of the 9 hemodialysis patients is shown in Figure 1. The endogenous Pco increased after dialysis in 8 of the subjects and remained constant in the ninth, with an average increase of 13.7+ 4.6% (P <0.05). The estimated RBC survival predialysis was 70±9 days and fell to61±9 days immediately postdi alysis.
The endogenous Pco of 8 healthy subjects averaged 156±0.33 ppm and147±0.14 ppm at12:30 PM and 4: 30 PM, and the respective rBC survivals were 132+ 11 and 140+ 15 days(P >0.20).
DISCUSSION
The conventional method [4-6] of measuring Vco for RBC survival studies requires that the subject rebreathe for a period of several hours into a closed system, consist-ing of a hood that encloses the head, an air circulating device, a cO, absorber and an O, delivery system. Mea surements of the rate of rise of the blood co concentra-tion and the total heme pool are used to calculate Vco and RBC survival. Theoretically this complex technique should yield very accurate Vco measurements; however in practice, accuracy is limited by the small rise in blood Co(about 10% in normal subjects) that occurs during the 4-hr rebreathing period. Thus, relatively small errors in blood CO measurements can lead to large errors in the calculated Vco.
We recently validated the use of a markedly simplified method in which Vco is estimated from measurements of end alveolar CO concentration. corrected for ambient CO [7]. This technique is based on several assumptions First, it is assumed that sedentary individuals have a relatively constant alveolar ventilation/kg: hence, the simple determination of alveolar CO concentration can be substituted for the complex measurement of total CO excretion rate. Second, the fraction of alveolar co that is of environmental ongin can be determined with a device that equilibrates with atmospheric CO at roughly the same rate as does the subject (1/2 time of 4.5 hr in healthy subjects)[8]. Subtraction of the equilibrator Pco from alveolar Pco yields the endogenous Pco, an indicator of co. The errors involved with the equilibrator become greater, the higher and more fluctuant the ambient Pco. Lastly, the blood volume/kg is assumed to be constant between individuals; hence, the hemoglobin pool size becomes a direct function of the blood hemoglobin con-centration. Despite these assumptions, we found that the mean and coefficient of variation of RBC survival in 32 healthy subjects compared favorably with that reported for the far more complicated rebreathing technique [9].
Based on endogenous Pco measurements, the RBC survival of 9 subjects on chronic hemodialysis averaged 70 ± 9 days when measured immediately pre-hemodialysis at about 12:30 pm. Since there appears to be a circadian rhythm to RBC tumover [10], comparison of this value with that of normal subjects requires attention to the time of day at which the measurement was obtained. Our previous study of 32 healthy subjects using endogenous Pco measurements showed a mean survival of 101 ± 3.5 days when measured at 8 am and 139 ± 10 days at 4 pm [7]. In the present study, we found the RBC survival of 8 healthy subjects at 12:30 pm to be 140 days. Thus, prior to hemodialysis the mean RBC survival of our patients was reduced to about 50% of normal, a somewhat greater reduction than has been observed in most previous studies of RBC survival in dialysis patients using 51Cr- or DF3232P-labeled RBC[11-13]. The greater survival when assessed with the labeling techniques is not surprising since these methods do not reflect ineffective erythro poiesis as does CO. In addition, if RBCs with differen life expectancies enter the blood, cells with longer life spans are disproportionately present at any moment and hence, these long-lived cells are disproportionately la beled. In contrast, CO measurements reflect the average life span of all cells entering the circulation plus loss through ineffective erythropoiesis.
The major goal of this study was to determine via endogenous Pco measurements, if the hemodialysis pro-cedure, per se, acutely altered rBC turnover The accu racy of this application of the CO technique requires that alveolar ventilation and total body heme remain constant during the hemodialysis procedure. older studies showed that hemodialysis induced hypoventilation as evidenced by a decreasing Po, [14-16]. However, this hypoventila tion appeared to be attributable to the use of acetate in the dialyzing fluid [16] and/or bio- incompatible membranes such as cuprophane [14]. with the technique employed in the present report, i. e, the use of bicarbonate in place of acetate and bio-compatible cellulose acetate membranes De Backer et al. reported that the Pcoz and Poz remained virtually constant during hemodialysis [17]. It also should be noted that due to the 4, 5 hr 1/2 time of co in the body, marked alterations in alveolar ventilation are required to bring about appreciable changes in endoge nous Pco over the 4-hr hemodialysis run. For example, it can be calculated that alveolar ventilation would have to be reduced by about 33% throughout the entire 4-hr pe riod to produce a 15% increase in Pco. Hypoventilation of this magnitude should be readily detected clinically.
While blood volume may be altered during dialysis only small amounts of blood(about 5 ml)are lost during the procedure. Since the production of CO is dependent upon the heme mass, but independent of the blood vo ume, shifts in fluid volume during hemodialysis should not influence the results of CO measurements. Lastly, in the controlled, smoke-free environment of the hospital ambient CO remains relatively low and constant. Thus the major variable in the rbC survival calculation over the short time period of hemodialysis should be Co pro duction which will be accurately assessed via the highly reproducible measurement of end alveolar Pco.
Endogenous Pco increased in 8 of 9 subjects during the 4-hr hemodialysis(12: 30 PM to 4: 30 PM) and remained constant in the ninth subject (P <0.05), with an average increase of 14%(see Fig. 1). Because the endogenous c co of healthy subjects was previously observed to de-P line by about 20% between 8 AM and 4 PM [7] studies were carried out to better define the alteration in endoge nous Pco expected between 12: 30 PM and 4: 30 PM, in the absence of dialysis. In 8 subjects, endogenous Pco dropped by an average of 6% over this time period. Thus during the hemodialysis procedure, endogenous Pco in-creased by a total of about 20% and rBC survival de creased by about 20% compared to what otherwise would have been expected during the time interval of the dialy sis run. This slightly increased rate of RBC destruction occurring intermittently during hemodialysis should have little impact on the patients' hemoglobin concentrations.
A number of studies have demonstrated diminished RBC survival in patients with chronic renal failure [1013]. In the early days of dialysis, many reports docu mented relatively massive RBC destruction during hemo-dialysis due to exposure to excessively warm [181] hypotonic [19] dialysate, or to excessive concentrations of nitrate [20], chloramine [21], or copper [22]. Formalin sensitivity [23] and obstruction of the extracorporeal cir-cuit [24] also were reported to cause hemolysis during hemodialysis.
With the present, careful monitoring of the dialysate and the dialysis conditions, hemolysis secondary to the hemodialysis procedure is considered to be negligible [25, 26]. However, the ability to detect low grade, acute increases in RBC turnover is dependent upon the sensitiv.ity of the technique employed to assess RBC destruction Of particular interest was the inability of Lerner and coworkers [27] to demonstrate that hemodialysis en-hanced rbc breakdown when assessed by conventional measurements of Vco obtained with the rebreathing tech-nique. These workers found that the Vo co(umol/mmo total body heme/day)of 8 subjects averaged 14.0+7.9 between dialyses and 16.4+5.7 during the dialysis pro-cedure(P>0.05). However, the average percent differ ence(independent of sign) between the intra- and inter-dialysis measurements of an individual was 62%. Thus, it is not surprising that this study was unable to detect the 14% increase in endogenous Pco that we observed in patients undergoing hemodialysis.
We conclude from this study of hemodialysis patients that measurements of endogenous Pco are likely to pro-vide the most accurate available means of assessing the influence of any acute process on RBC turnover. Addi-tional advantages of this non-invasive technique are tha it demands little patient cooperation and is technically simpler than any other measurement of RBC turnover requiring only a few minutes of technician time for each survival measurement.
ACKNOWLEDGMENTS
Supported in part by NIDDKD ROl DK 13309-25 and the Department of veterans Affairs. The authors thank Dr. David M. and Martha Sims for their assistance.
REFERENCES
1. Shemin D; Rittenberg D: The life span of the human red blood cell. J Biol Chem166627-636.1946.
2. Penner JA: Investigation of erythrocyte tumover with selenium 75labeled methionine, J Lab Clin Med 67: 427-438. 1966.
3. Ebaugh FJ Jr. Emerson CP Rose JF: The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination of red cell survival in vivo. J Clin Invest 32: 1260-1276, 1953.
4. Coburn RF, Blakemore wS, Forster RE: Endogenous carbon monox ide production in man. J Clin Invest 42: 1172-1178. 1963.
5. Coburn RF, Williams WJ, Kahn SB: Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest 45: 460-468,1966.
6. Landaw SA, Callahan EW. Schmid R: Catabolism of heme in vivo. J Clin Invest 49: 914-925. 1970.
7. Strocchi A Schwartz S, Ellefson M, Engel RR, Medina A, Levit MD A simple carbon monoxide breath test to estimate erythrocyte turm-over J Lab Clin Med 120: 392-399, 1992.
8. God in G, Shephard RJ: On the course of carbon monoxide uptake and relapse. Respiration 29: 317-329, 1972.
9. Lundh B, Cavallin-Stahl E, Mercke C: Heme catabolism, carbon mon-oxide production and red cell survival in anemia. Acta Med Scand197:|61-l71,1975.
10. Mercke C, Cavallin-Stahl E, Lundh B: Diurnal variation in endoge nous production of carbon monoxide. Acta Med Scand 198: 161-1641975.
11. Hocken AG: Hemolysis in chronie renal failure. Nephron 32: 28-311982.
12. Shaw AB: Haemolysis in chronic renal failure. Br Med J( Clin Res) 2:213-216.1967.
13. Stewart JH: Haemolytic anaemia in acute and chronic renal failure. QJ Med36:85-105,1967.
14. Craddock PR, Fehr J, Brigham KL, Kronenberg R, Jacobs HS: Com-plement and leucocyte-mediated pulmonary dysfunction in hemodialy sis. N Engl J Med 296: 769-374, 1968.
15. Aurigemma NM, Feldman NT, Gottlieb M, Ingram RH Jr, Lazarus JM, Lowrie EG: Arterial oxygenation during hemodialysis N Engl J Mad29:871873.1977.
16. Tolchin N, Roberts JL, Lewis EJ: Respiratory gas exchange by high efficiency hemodialyzers Nephron 21: 137-145, 1978.
17. De Backer WA, verpooten GA, Borgonjon DJ, Vermeire PA. Lins RR, De Broe ME: Hypoxemia during hemodialysis: Effects of differ ent membranes and dialysate compositions. Kidney 23(15): 738-7431983.
18. Berkes SL, Kahn Sl, Chazan JA, Garella S: Prolonged hemolysis from verheated dialysate. Ann Intern Med 83: 363-364, 1975.
19. Said R, Quintanilla A, Levin H, Ivanovich P: Acute hemolysis due to profound hypo-osmolality. A complication of hemodialysis, I Dialys 1:447-4521977.
20. Carlson DJ, Shapiro FL: Methemoglobinemia from well water nitrates A complication of home dialysis. Ann Intern Med 73: 757-759, 1970.
21. Higgins MR, Gace M, Ulan RA, Silverberg DS, Bettcher KB, Dosse tor IB: Anemia in hemodialysis patients. Arch Intern Med 137: 172176,1977.
22. Manzler AD, Schreiner AW: Copper-induced acute hemolytic anemia A new complication of hemodialysis. Ann Intern Med 73: 409412,1970.
23. Kachny WD. Miller GE, white WL: Relationship between dialyzer reuse and the presence of anti- N-like antibodies in chronic hemodialy sis patients. Kidney Int 12: 59-65, 1977.
24. Francos GC, Burke JF Jr, Besarb A, Martinez J, Kirkwood RG Hummel LA: An unsuspected cause of acute hemolysis during hemo-dialysis. Trans Am Soe Artif Intern Organs 24: 140-145, 1983.
25. Lerner R, Werner B, Asaba H. Temstedt B, Elmqvist E: Assessment of hemolysis in regular hemodialysis patients by measuring carbon monoxide production rate. Clin Nephrol 20: 239-243. 1983.
26. Dhaene M, Bulbis B, Lietaer N, Gammar N. Thayse C Ooms HA.Vanherweghem JL: Red blood cell destruction in single-needle dialy sis. Clin Nephrol 31: 327-331. 1989.



