Effect of Hemmodialysis on the Red Blood Cell Life Span in Patients with End-Stage Kidney Disease
Abstract:The aim of this study was to use a CO breath test to investigate hemodialysis effects on red blood cell lifespan in patients with chronic kidney disease. A cohort of 17 non‐smoking men with end‐stage kidney disease undergoing hemodialysis via a polysulfone dialysis membrane (as opposed to a traditional cellulose acetate membrane) were subjected to a repeated Levitt's CO breath test to compare red blood cell lifespan before vs. after dialysis. None of the patients showed significant fluctuations in endogenous CO concentration during the dialysis procedure. The mean red blood cell lifespan was 66.0 ± 31.0 days before dialysis and 72.0 ± 26.0 days after dialysis, with no significant difference between the assessment time points (P > 0.05). In conclusion, dialysis using a polysulfone membrane did not appear to disrupt red blood cells or reduce their lifespan in patients with end‐stage kidney disease.Key Words: Biocompatibility, Chronic kidney disease, Hemodialysis, Levitts Co breath test, Red blood cell lifespan.
Anemia is a common complication and important rognostic factor of end-stage kidney disease (ESKD)(1). Data from the phase I DOPPS study in the Usa have indicated that chronic kidney dis ease(CKD)patients with hemoglobin levels below 110 gL have a shorter survival time than those with higher levels(2). Anemia is thought to develop as a result of multiple processes including inadequate erythropoietin production, suppression of bone mar row function by uremic toxins, iron deficiency blood component loss, susceptibility to infection underlying hematological disease, hyperparathyroid-ism, nutritional deficits, and an abnormally short red blood cell(RBC) lifespan(3, 4) Some studies have suggested that maintenance hemodialysis(HD), which is a common treatment for patients with ESKD, may reduce average RBC survival because of compression and twisting of cells in hd blood lines(5-7). Indeed Medina et al.observed immediate surges in dead rbc debris fter HD with slightly reduced averaged rBC life span post-HD, compared with pre-HD values (7)
In light of these observations, there is a concerning possibility that long-term dialysis may aggravate CKD associated anemia. In the present study, we used Levitt's co breath test to measure and compare rbc lifespan before vs. after HD in patients with ESKD.
PATIENTS AND METHODS
Study subjects
Patients with ESKD(N= 17) undergoing mainte nance hd were recruited to this study from the Department of Nephrology, Shenzhen Nanshan Hospital, Guangdong Medical School, Shenzhen, China. All of the patients were non-smoking men, with a mean age of 48+ 12 years old (range,
29-71 years). We excluded subjects with chronic ung disease which would affect endogenous CO detection and the calculation of RBC lifespan. A study with a self-controlled design was conducted to minimize the influence of potential chronic lung dis ease subjects. Patient kidney disease diagnoses included chronic glomerulonephritis (N=7), dia betic nephropathy (N=6), polycystic kidney (N= 1), and unknown(N= 3). The mean number of years on HD in these patients was 6.5+.9 years ange, 1-19 years). The patients underwent HD three times per week, with 12 patients commencing HD at 8: 30 am and 5 patients ce ng HD at 1:00pm.
All of the patients provided written informed con sent to participate in the study. The study protocol was approved by the Institutional review board of Nanshan Hospital (NO. 2018092503).
Hemodialy
HD was performed in disposable BLS514SD dia-lyzers(Sorin Group Italia S.R. L, Mirandola, Italy)
with a 35-um-thick polysulfone dialysis membrane (internal pore diameter, 200 um). The ultrafiltra tion, blood flow, and dialysate flow rates were 26 mL/h mmHg, 200-300 mL/min, and 500 mL/min The dialysate composition was as follows: Na+139. 8 mmol/L; K+: 2.0 mmol/L;Ca+: 1.5 mmol/L;Mg2+ 0.50 mmol/L;Cl-:106.8mmol/L;CH3COO-: 4.0 mmol/L; and HCO3-: 35.0 mmol/L The duration of hd per session was 4 h
Levitts Co breath test of RBC lifespan
RBC lifespan is an objective index of RBC clear-ance that refers to the survival period of RBCs which enter circulation after having matured in bone marrow. RBCs have a typical lifespan of
120 days in healthy adult humans, but the RBC life span is dramatically shortened in patients with ESKD. The Levitt CO breath test principle, which was introduced in 1992( 8), has been applied for measuring steady-state RBC status assessment as well as for monitoring short-term, dynamic RBC clearance rate (9). Endogenous CO is produced mainly during heme catabolism after RBCs are cleared. Therefore, RBC lifespan can be estimated by dividing the total CO release from RBCs by the daily Co release. The Levitt formula is as follows:
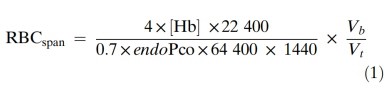
The numerator of the formula represents the total CO release from hemoglobin within the body where the multiplier of four indicates that 1 mol of hemoglobin produces 4 mol of CO after upon deg-radation, [Hb is the hemoglobin concentration (g/mL), 22 400 is the standardized molar volume (mL), and Vb is the blood volume of the body (mL) The denominator of the formula represents the daily Co release, where the 0.7 multiplier approxi-mates the ratio of endogenous Co produced by hemoglobin turnover, 64 400 is the molecular weight of hemoglobin. 1440 is the number of minutes in a day(min), endopco is the partial pres sure of endogenous co(difference between the partial pressure of alveolar CO and air CO, ppm) and Vi is the resting alveolar ventilation rate (mL/min). Because Vb and v both tend to vary directly with alveolar surface area and have roughly similar magnitudes if represented by mL and mL/min, respectively, they can be mutually canceled out from the formula above. The levitt formula can thus be simplified to:
RBCspan=(Hb 1380)/endopco (2)
As above, [Hb] is the hemoglobin concentration (g/mL) and endopco is the partial pressure of endogenous co. while 1380 is the product of the numerical variables in the first equation. Hence RBC lifespan values derived from the levitt for-mula are based on the parameters of [Hb] and endoPco.
Measurement protocol
Alveolar air samples collected 5 min before and then 10 min 2h. and 4 h after hd was started were used to measure the rBc lifespan. Venous blood samples collected 5 min before hd and at the com-pletion of HD were submitted for conventional hemoglobin concentration tests.
For alveolar air sample collection, patients were seated or lying in a supine position with the mouth unit of a disposable air bag in their mouth. First each patient took a deep breath(away from the air bag), followed by breath-holding for 10 S, and then he or she exhaled into the air bag until it was full The exhale step was repeated with breaks that lasted for several seconds until the air bag was full he air collection device contained a three-V switch that connected the mouth unit. alveolar bag, and cavity air bag. The alveolar air bag and cavity air bag were made from aluminum with vol-umes of 1500 and 300 mL, respectively. The cavity air bag was allowed to fill first during breathing out and this portion of air was considered to be largely from the physiological dead space and thus dis carded. Subsequent exhaled air was directed into the alveolar air bag. While the alveolar air was being collected, environmental air was also collected with a pump. Air samples were stored at room temperature for no more than 5 days before the measurements were completed.
Finally, RBC lifespan was determined with an ELS Tester machine( Shenzhen Xianya Biotechnol ogy Co, Ltd. Shenzhen, China), which measures endogenous CO by non-dispersive infrared compar-ison of air vs alveolar Co content and then calcu lates RBC lifespan using the Levitt formula Operation of this instrument involved a simple three-step protocol: (i) addition of alveolar and environmental samples; (ii) inputting of[ Hb] information; and (ii) pressing a start measure-ment button The measurements and calculations for each assessment were completed within 15 min.
Statistical analysis
Paired t tests were performed for within-group comparisons of RBC lifespan before vs. after HD. SPSS 13.0(sPSS for Windows, version 13, SPSS Chicago, IL, USA)was used for statistical analysis Data are expressed as means t standard deviations (SDs)and P<0.05 was considered significant.
RESULTS
Exhaled endogenous CO
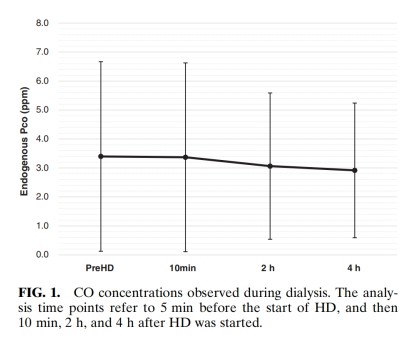
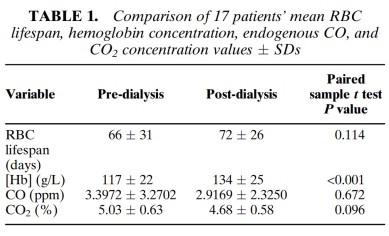
Endogenous CO content during HD remained statistically stable over the four measurement time points(Fig. 1)and between the pre-HD and final post-HD measures (Table 1).
RBC lifespan
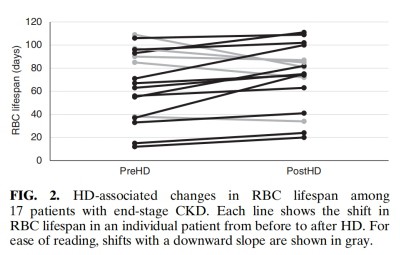
Overall, mean RBC lifespan was statistically simi-lar before vs. after HD (Table 1). Regarding abso-lute values determined relative to each patient's own pre-HD values, 12 patients had an increased RBC lifespan after HD and five patients had a decreased RBC lifespan(Fig. 2). Re-performing the calculations as in Medina et al. ' s study (7)(i. e, pre-HD [Hb] used in Levitt's formula for both pre-and post-HD calculations), the mean RBC lifespan remained statistically similar before( 66+ 31 days) vs. after(63+ 26 days)after HD.
DISCUSSION
The present study showed that HD carried out with a polysulfone membrane did not alter RBC lifespan significantly in patients with ESKD. CO nd co, levels in exhaled air remained stead across pre-HD and post-HD assessments, with none of the 17 patients showing any significant fluctua-tions in endogenous co concentration during the dialysis procedure. Meanwhile, we found that hemo-globin concentrations in blood increased signifi cantly following HD relative to pre-HD levels.
Traditional methods of assessing RBC lifespan with radioactive labeling take weeks, or even some mes months, to complete and thus are applicable for steady-state measurements, but cannot be used to probe transient RBC clearance during HD. For example, using traditional radioactive chromium labeling, Frederiek et al. (10) reported that median RBC lifespan was significantly decreased, by 20% in long-term HD patients compared with levels observed in healthy controls. To overcome the chal-lenge of determining dynamic RBC clearance rates evitts research group introduced the co breath test in 1992(9), a methodological approach based on the principle that endogenous CO released in expiration is released mainly as a producer of heme catabolism following degraded rBC clearance. In a 1994 study of nine patients with ESKD, using an earlier protocol and machine, Medina et al.(7) found that endogenous co concentrations increased by 14% on average during HD, while mean pre-HD and post-HD RBC lifespan was significantly reduced from70士9 days to61±9days.That study provided strong evidence of a destructive influence of hd on rbcs and thus evidence of hd contributing to a in patients receiving long-term HD. Conversely, in the present study of 17 ESKD patients, the Levitt Co breath test showed that hd did not change endogenous co concentrations or RBC lifespan values, suggesting that HD, as performed in our patient population was not disruptive to RBC survival.
The discrepancy between our results and medina et al. aforementioned results might be attributable to three major differences between these studies Firstly, methodologically, Medina et al. (7)used pre-HD hemoglobin concentration values in the Levitt formula(Eq.(2)) to calculate RBC lifespan both before and after HD. In contrast, we used pre-HD and post-HD hemoglobin values for pre-HD and post-HD RBC lifespan value calculations respectively. It is our view that pre-HD concentra tions should not be used to calculate post-HD RBC ifespan because hemoglobin concentrations tend to increase after dialytic dehydration. Notwithstanding when we re-calculated RBC lifespan values using the same method used by Medina et al. (7) with our data, RBC lifespans remained not significantly dif ferent pre- vS. post-HD. Thus, the difference in calculation methodology cannot explain, at least not Is a major factor, why the results between the two tudies were inconsistent
Second, we used a different dialysate protocol than was used by Medina et al.(7). In the present study, the dialysate did not contain glucose. Median et al. had added glucose to the dialysate to a con entration of 200 mg/dL. This difference may be meaningful given that dialysate ingredients can affect cellular activity. While concerns have been raised that HD may cause a large oxidative esponse that damages the rBC membran skeleton and, consequently, shortens RBC lucose can be used to increase dialysate dant activity, including glucose-6-phosphate dehy drogenase activity, which should, theoretically help to protect the cells(10). However, Medina et al. (7)observed more RBC degradation, not RBC protec-tion, relative to our present observations. Therefore, the presencelabsence of glucose does not appear to be a good explanation for the inconsistency of results between these two studies.
Finally, the dialysis instrument that was used to conduct the Hd to which patients in Medina et al.s study(7) were subjected was operated with a cellu-lose acetate dialysis membrane whereas the HD nstrument used with our patients was operated with a synthetic polysulfone membrane. Numerous stud-ies have shown that a synthetic membrane has sig nificantly better biocompatibility than natural cellulose (11-16).a bio incompatible membrane can activate inflammatory cells, resulting in the pro-duction of free radicals and reactive oxidant species that can damage RBCs and, ultimately, lead to microhemolysis (17-19). Therefore, it is possible that Medina et al.'s(7 observations of increased endogenous co concentrations and a shortened RBC lifespan due to HD were related to the bio incompatibility of the dialysis membrane used Thus, the effects of different dialysis instruments with different membrane biocompatibilities on CO levels, RBC lifespan, and related biochemical indi-ces should be compared.
CONCLUSION
In summary, in the present study employing a simple levitt co breath test protocol with an easy. to-operate analyzer machine, hd did not affect RBC lifespan significantly in patients with ESKD Previous observations of destructive effects of hd on RbCs may be related to the use of a dialysis mem brane material with unsatisfactory biocompatibility.
Acknowledgments: We would like to thank Seekya Biotechnology Ltd.( Shenzhen, China) for lending us an Els Tester co breath test device and thank staff in the Department of Nephrology, Nanshan Hospital(Shenzhen, China) for assisting with alveo-lar air sample collection. We thank Ellen Knapp, Phd,fromLiwenBianji,EdanzGroupChina(www.liwenbianji. cn/ac), for editing the English text of a preliminary draft of this manuscript. We thank Ann PowerSmith,Phd,ofWriteScienceRight(www.writescienceright. com) for providing professional sci-entific language editing of the final manuscript.
All authors contributed significantly to this work and are in agreement with the content of th manuscript.
The protocol for the research project was approved by the Nanshan Hospital Ethics Committee and
confirmed to conform with provisions of the declara tion of Helsinki(as revised in Tokyo 2004), Conflict of Interest: None
REFERENCES
1. KDOQI. Clinical practice guidelines and clinical practice rec-ommendations for anemia in chronic kidney disease Am Kidney Dis 2006: 47(5 Suppl 3): S11.
2. Robinson BM. Joffe MM. Berns JS. Pisoni RL. Port FK Feldman HI. Anemia and mortality in hemodialysis patients accounting for morbidity and treatment variables updated over time, Kidney Int 2005: 68: 2323-30.
3. Sato Y, Mizuguchi T, Shigenaga S et al. Shortened red blood cell lifespan is related to the dose of erythropoiesis-stimulating agents requirement in patients on hemodialysis Ther Apher dial 2012: 16: 522-8.
4. Tsagalis G. Renal anemia: a nephrologists view. Hippokratia 2011;15:3943.
5. Centers for disease Control and prevention multistate out-break of hemolysis in hemodialysis patients-Nebraska and Maryland. JAMA 1998 280: 1299-300.
6. Dutka P Guarding against hidden hemolysis during dialysis an overview, Nephro/ Nurs J 2008: 35: 45.
7. Medina A, Ellis C. Levitt MD. Use of alveolar carbon mon-oxide measurements to assess red blood cell survival in hemodialysis patients, Am J Hematol 1994: 46: 91-4.
8. Strocchi A, Schwartz S, Ellefson M, Engel RR, Medina A Levitt MD, a simple carbon monoxide breath test to esti-mate erythrocyte turnover. Lab Clin Med 1992: 120: 392-9.
9. Furne JK, Springfield JR, Ho SB, Levitt MD, Simplification of the end-alveolar carbon monoxide technique to assess erythrocyte survival. J Lab Clin Med 2003: 142: 52-7.
10. Vos fe. schollum JB. Coulter Cv. doyle TCA. Duffull SB Walker R. Red blood cell survival in long-term dialysis patients. AmJ Kidney Dis 2011: 58: 591-8.
11. Olszewska M, Bober J, Wiatrow J, Stepniewska J Dolegowska B, Chlubek D. The impact of hemodialysis on erythrocyte membrane cytoskeleton proteins. Postepy Hig Med dosw2015;69:165-75.
12. Cianciolo G, Stefoni S, Donati G et al. Intra- and post dialytic platelet activation and PDGF-AB release: cellulose diacetate vs Ifone membranes. Nephrol Dial Transplant 2001;16:1222-9.
13. Bour T, Vanholder R. Which dialyser membrane to choose?Nephrol Dial Transplant 2004: 19: 293-6.
14. Vienken J, Biocompatibility of dialysis membranes. Biomed Pharmacother 2006: 60: 472-3.
15. Young E. Dialysis dose, membrane type, and anemia control Am Kidney Dis 1998 32: S157-60.
16. Locatelli F, Mastrangelo F, Redaelli B et al. Effects of differ ent membranes and dialysis technologies on patient treat ment tolerance and nutritional parameters, Kidney h 1996;50:1293.
17. Wratten M. Tetta C. Ursini F, Sevanian A. Oxidant stress in hemodialysis: prevention and treatment strategies. Kidney Int 2000:58( Suppl76:s12632.
18. Borazan A, Aydemir S, Sert M, Yilmaz A, The effects of hemodialysis and peritoneal dialysis on serum homocysteine and C-reactive protein levels. Mediat Inflamm 2004: 13: 361-4.
19. Schwedler S. Schinzel R. Vaith P. Wanner C. Inflammation and advanced glycation end products in uremia: simple coex istence, potentiation or causal relationship? Kidney Int 2001:59( Suppl78)S32-6.



