Use of an oral stable isotope label to confirm variation…
Use of an oral stable isotope label to confirm variation in red blood cell mean age that influences HbA1c interpretation
Paramjit K. Khera Eric P. Smith Christopher J. Lindsell Mary Colleen Rogge Shannon Haggerty David A. Wagner Mary B. Palascak Shilpa Mehta Jacqueline M. Hibbert Clinton H. Joiner Robert S. Franco Robert M. Cohen
HbA1c is commonly used to monitor glycemic control. However, there is growing evidence that the relationship between HbA1c and mean blood glucose (MBG) is influenced by variation in red blood cell (RBC) lifespan in hematologically normal individuals. Correction of HbA1c for mean RBC age (M RBC) requires a noninvasive, accurate, and affordable method to measure RBC survival. In this study, we evaluated whether a stable isotope approach would satisfy these requirements. RBC lifespan andM RBC were determined in a group of nine hematologically normal diabetic and nondiabetic subjects using oral 15N‐glycine to label heme in an age cohort of RBC. TheM RBC was 58.7 ± 9.1 (2SD) days and RBC lifespan was 106 ± 21 (2SD) days. This degree of variation (±15–20%) is consistent with previous studies using other techniques. In a subset of seven subjects, M RBC determined with the biotin label technique were available from approximately five years prior, and strongly correlated with the stable isotope values (R 2 = 0.79). This study suggests that the M RBC is stable over time but varies substantially among individuals, and supports the importance of its variation in HbA1c interpretation. The characteristics of the stable isotope method support its suitability for studies to directly evaluate the impact of variation in M RBC on the interpretation of HbA1c. Am. J. Hematol. 90:50–55, 2015. © 2014 Wiley Periodicals, Inc.
Introduction
It is widely accepted that Hemoglobin A1c (HbA1c) is equivalent to mean blood glucose (MBG), and it is used routinely to monitor blood glucose control in diabetes 1. In addition, HbA1c has been a critical measure in studies showing that tight glycemic control reduces microvascular 2 and macrovascular 3, 4 complications. Nonetheless, there is growing evidence that MBG may not be the only factor that influences HbA1c 5.
HbA1c depends on the rate at which hemoglobin is glycated 6 and the time for glycation 7. The rate is dependent on MBG 8, but may be affected by other factors including temperature, pH 6, 9, phosphate, and inhibitors present in renal failure 10. There may also be deglycation of lysine by the enzyme fructosamine‐3‐kinase 11 which is a consideration for total glycated hemoglobin but not for HbA1c per se . The time for glycation is equal to the M RBC. The assumption is made that other than MBG there is little variation in these factors among individuals, and therefore glycation reflects only MBG 6, 7.
The assumption that M RBC is uniform in the absence of clinical hemolysis is called into doubt by studies employing a variety of methods 12-14 to measure RBC lifespan, including recent investigations using a precise biotin RBC label 12. RBC lifespan in hematologically normal people, with and without diabetes, has varied by as much as 30% (±15%). This range is substantial since HbA1c is expected to be directly proportional to M RBC and only a range of 5–8% HbA1c determines most clinical care decisions. These studies suggest that for optimum care many people with diabetes require a correction to HbA1c based on RBC lifespan 12, 15-17.
Methods for measuring red cell lifespan use either a population label or an age cohort label18. In the former, a representative population of RBC that contains cells of all ages are labeled ex vivo and reinfused. The time‐dependent decrease in labeled cells determines RBC lifespan. Examples of this approach include the standard radioactive clinical label, 51Cr19, and the more precise research label, biotin 20. In contrast, the use of a metabolic precursor that can be administered orally and biosynthetically incorporated into heme results in a cohort label 21. Since no ex vivo labeling is required, there are no associated laboratory costs for labeling, no possibility of error in the identification of material for re‐infusion, and no potential for bacterial contamination. In addition, the advantage of a cohort label is that all the RBCs produced during a defined time period are included in the measurement of lifespan. This is in contrast to a population label in which selection pressures may be applied to the RBCs prior to labeling 22.
In the current study, our goal was to further develop a stable isotope based cohort measure of RBC lifespan (expressed as M RBC) that would facilitate the assessment of RBC survival as a clinically significant confounder of HbA1c. A precursor of heme containing a stable, non‐radioactive isotope (15N‐glycine) was administered orally; this results in enrichment of 15N in an age cohort of newly produced RBC. As the four nitrogen atoms in heme are derived from four glycine precursors, adequate labeling is achieved at a reasonable dose of 15N‐glycine. Although this approach was applied in the 1940s and was used through the 1970s on a modest scale, the method was largely replaced by 51Cr because of the expensive and cumbersome mass spectrometry of those eras and the cost of the stable isotope 14. However, 15N‐glycine of high specific activity has become sufficiently inexpensive for more widespread use in metabolic studies. Quantitation of heme 15N/14N was performed by a commercial laboratory after heme extraction from a batch of frozen blood samples. In a subgroup of seven subjects, we compared M RBCdetermined with the stable isotope method with values obtained approximately five years previously using the biotin population label. The correlation between the two methods was good, although the M RBC values were higher for the stable isotope method.
Methods
Subjects. Ten adults with (1 M, 2 F) and without (2 M, 5 F) diabetes (see Supporting Information Table 1) participated, including seven with (1 M, 2 F) and without (1 M, 3 F) diabetes previously studied using the biotin label technique 12. Subjects with diabetes maintained a stable level of control. The following were exclusion criteria: baseline serum creatinine > 1.5 mg/dL, urine albumin >200 μg/min (timed collection) or > 179 μg/mg creatinine (spot collection), transaminases [alanine aminotransferase (ALT) shown in Supporting Information Table 1; asparatate aminotransferase not shown] more than three times the upper limit of normal, heart failure greater than or equal to New York Heart Association stage III, hematocrit <34%, reticulocyte count >2%, evidence of hemoglobinopathy (high performance liquid chromatography), history of gastrointestinal blood loss, pregnancy, blood bank donation or transfusion in previous 4 months, uncontrolled thyroid disease, active infection, and underlying illness known to be associated with body wasting (e.g., malignancies or tuberculosis). Nondiabetic subjects were recruited from the general population. The protocol was approved by the University of Cincinnati Institutional Review Board, and informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Subject 3 withdrew from the study for reasons unrelated to any side effect and was not included in the analysis.
Reagents
15N‐Glycine (98%) ( H215NCH2COOH), Catalog # NLM‐202, and 13C‐glycine (99%) (H2NCH213COOH), Catalog # CLM‐422‐PK) were obtained from Cambridge Isotope Laboratories, Inc. Andover, MA.
Experimental design
After collection of baseline blood samples, 15N‐glycine or 13C‐glycine was given orally on day 0 dissolved in 30 mL of H2O. Subject 1 received 2 g of each in three equal hourly doses. All other subjects received a single 2 g dose of 15N‐glycine only. Blood samples were drawn on Day 3 or 4 and weeks 1, 2, 3, 4, 6, 8, 10, 12, 14, 15, 16, 17, 18, 20, 23, and 26. These were stored at −80°C for heme isolation in a single batch at the end of the experiment.
Analytical procedure
Heme was extracted from whole blood hemolysates using a method modified from that of Egyed 23. Ten milliliters of acetone were added dropwise to 1 mL of blood with constant mixing. After 30 min at room temperature, the precipitate was pelleted and the supernatant was discarded. The pellet was vortexed in 5 mL ethylacetate‐glacial acetic acid (3:1), gravity filtered (Whatman 2 filter paper) to remove the undissolved protein, brought up to 50 mL with deionized distilled water (ddH2O), and shaken vigorously to precipitate heme. The heme was pelleted (30 min at 2,800 rpm), washed sequentially with 50 mL and 2 mL of ddH2O, lyophilized, and stored at −80°C. All of the samples for each subject were run in a single batch. The 15N/14N ratio in heme was measured by a commercial laboratory (Metabolic Solutions, Inc., Nashua, NH) using a Europa Scientific 20/20 gas isotope ratio mass spectrometer 24. The baseline 15N/14N and 13C/12C ratios were subtracted from ratios obtained after administration of stable isotope to calculate the atom percent excess (APE). A relative atom percent excess (%APE) was derived for each time point using the maximum value as 100%.
RBC survival analysis
The %APE increased during the days following ingestion of stable isotope as cells that were at various stages of normoblast differentiation and, therefore, synthesizing hemoglobin at the time of labeling 14, 18, 25 were released. Since the rate of heme synthesis in these cells at various stages of development varies, a given increase in %APE does not necessarily represent the same number of cells released over that time period 14, 18, 26. Because of that variability, for this analysis we selected the time at which the %APE reached 50% of the maximum value as the starting point for all calculations. The end time was defined as the time when the rate of change of %APE had decreased to less than 0.5% per day.
The %APE did not return completely to baseline, but instead reached a near steady residual value between about 15 and 25%. This is most likely due to recycling of stable isotope 14,26-29 into heme in RBC that was not part of the original age cohort. We examined the effects of the following three possibilities for the origin of the residual %APE: (1) a linear model that postulates a gradual increase in noncohort heme, (2) a “mirror image” model that corresponds to all of the excess coming from the recycling of cohort red cells that have reached the end of their lifespan, and (3) a constant (except in the first 20 days) model that corresponds to all the excess being due to other proteins with relatively fast turnover. We corrected the %APE at each time point according to the assumed model and for this analysis approximated the M RBC by dividing the median survival by two. The values for a typical experiment were as follows: uncorrected: 60 days; linear: 58 days; mirror image: 59 days; constant: 56.5 days. Thus the half median survival value is quite insensitive to the models tested. For this manuscript, we used the linear model to correct the data.
To evaluate the effect of RBC survival on HbA1c, we chose M RBC over other measures of RBC survival 27, 29-31. For each RBC survival curve for an age cohort, the M RBC was calculated from a life table analysis. At steady state, this analysis enables the conversion of a single measured cohort survival curve to a mean age for all the RBC in the circulation (Fig.1C, Supporting Information Table II and Supporting Information text).
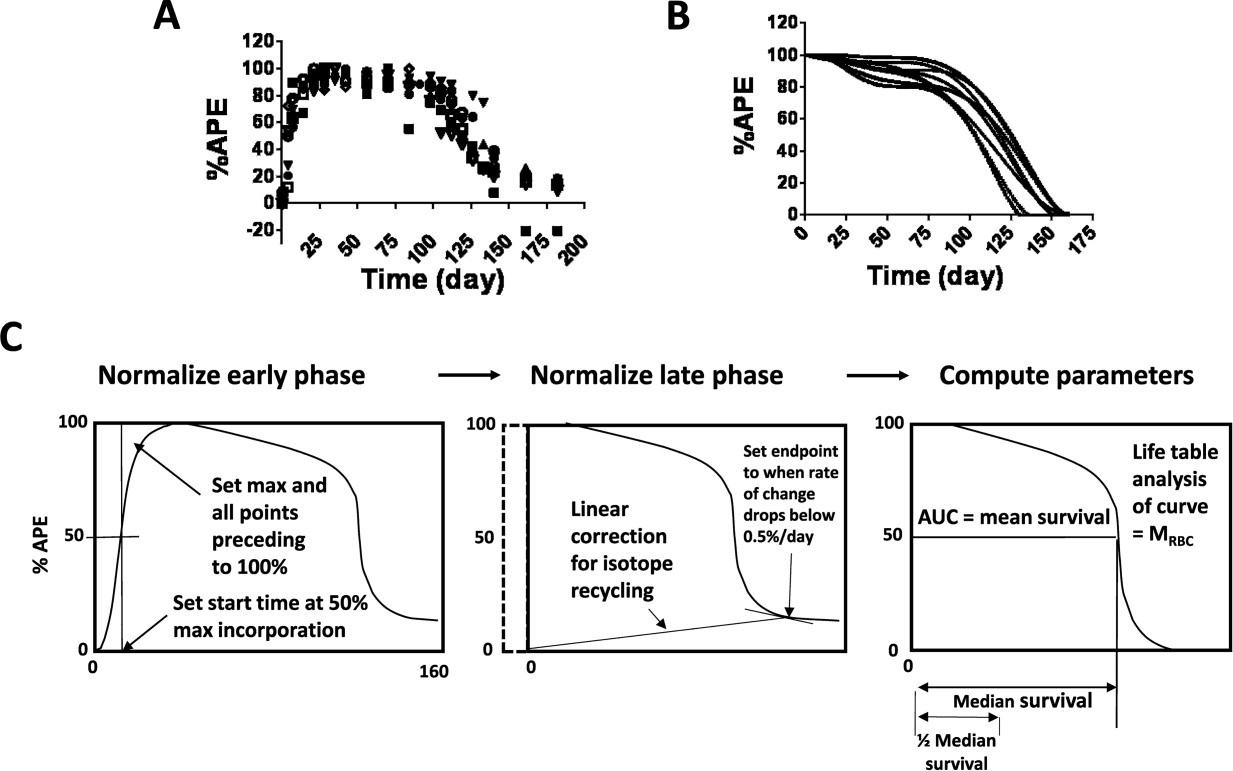
Figure 1
RBC 15N/14N heme after oral administration of 15N‐glycine in nine subjects and graphical display of normalization with comparison of different measures of RBC survival. The original data (A) are shown on the left top row and the best fit RBC survival function is plotted on the right top row (B). Each time point is expressed as % of maximum atom percent excess (%APE). C: The %APE curve was normalized by setting the maximum value to 100%, setting the time at which 50% of maximum incorporation was reached as the start time, setting the time at which the slope reached a value less than 0.5%/day as the end time, and assuming a linear increase in the portion of the %APE due to recycling of the stable isotope. In addition, time points between the start time and the maximum value were set to 100% and the curve was forced to be monotonically decreasing. Measures of RBC survival derived from the data included mean red cell survival (M RBC) (from a life table analysis of the curve), mean survival (from area under the curve, (AUC)), median survival as shown, and one half median survival.
Results
Dose and dosing schedule
We first confirmed a dose and dosing schedule for 15N‐ or 13C‐glycine based on previous experience 21, 32, 33. In the first subject, there was significant enrichment of %APE for both15N and 13C after simultaneous oral ingestion of equal amounts of 15N‐glycine and 13C‐glycine (2 g divided into 3 hourly doses), with no apparent advantage for the more expensive 13C‐glycine. The second subject ingested a single dose of 2 g 15N‐glycine and showed that divided doses are not necessary. Therefore, the remaining subjects were given 2 g of 15N‐glycine orally as one dose.
RBC survival
Figure 1 shows 15N %APE survival curves before (A) and after (B) normalization for the nine subjects (refer to Supporting Information Fig. 1 for individual survival curves for each of the nine subjects). For most subjects, the %APE had the following several distinct phases: (1) a rapid increase that started immediately after administration of 15N‐glycine and had a halftime of 2.8–6.3 days and a maximum value at 20–25 days, (2) a plateau or slightly decreasing period from 20–25 days to approximately 100 days, (3) a rapidly decreasing period from approximately 100 to 150 days, and (4) a level or slowly decreasing period after 150 days with a value between 15 and 25%. Two subjects (no. 4 and no. 9) demonstrated a decrease in %APE prior to reaching the plateau phase. This was due to a distinct maximum at about 25 days after labeling, and implies the presence of a minor population of RBC with very short survival. Figure 1C depicts the steps used to normalize the APE vs. time curves and shows the various measures of RBC survival derived from the data. (See Supporting Information for a detailed description of the normalization process and a worked example depicting normalization of the survival curve for Subject no. 8.).
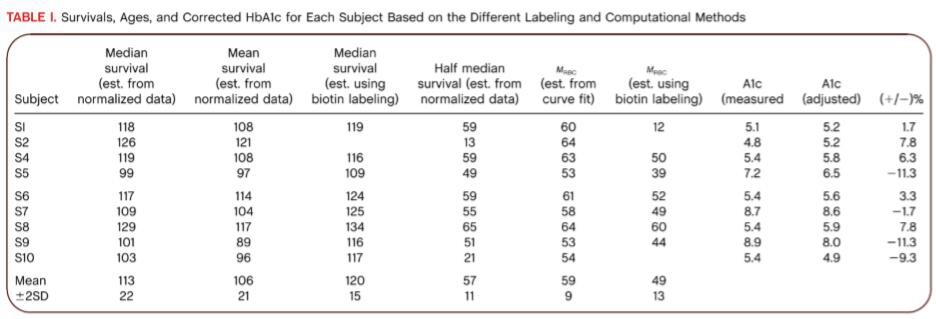
The stable isotope label derived median RBC survival was 113 ± 22 (2SD) days (Table 1). Mean survival was somewhat shorter, consistent with a small component of short‐lived cells. The M RBC calculated from the life table approach was 59 ± 9 (2SD) days and the adjusted HbA1c [(M RBC × mean M RBC)/M RBC) revealed as much as ∼11% difference between measured and corrected. Figure 2A shows the excellent correlation (R 2 = 0.95) between theM RBC calculated from the life table approach and the half median survival. This correlation suggests that RBC lifespan for these hematologically normal subjects has a near normal distribution and that for clinical purposes the simpler half median value may be adequate. Use of the half median value would have the following advantages: (1) The median survival is derived from only the starting time and the time required for the fitted normalized survival curve to decrease to 50% of the maximum value. The more complex life table calculations 22 for M RBC are not required. (2) Since the mid portion of the curve is less important for the median survival calculation, fewer time points during the plateau period may be required. (3) Both a median survival that corresponds to the commonly used and understood lifespan and a half median survival, that corresponds to mean age, are derived. (4) The median survival may be useful for other applications in which the lifespan rather than mean age is the more appropriate variable.
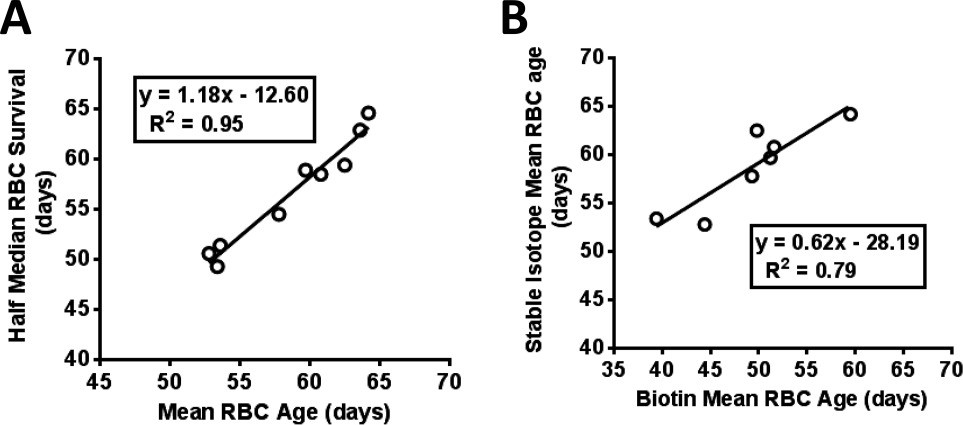
Figure 2
Correlation analysis. A: There is excellent correlation (R 2 = 0.95) observed between two modes of analysis, mean red cell survival (M RBC) and half median survival, on the stable isotope data, (B) High correlation (R 2 = 0.79) between theM RBC values determined by the biotin compared to stable isotope method.
Table 1. Survivals, Ages, and Corrected HbA1c for Each Subject Based on the Different Labeling and Computational Methods
Correlation with biotin method
A previous study from our group determined M RBC using the ex vivo biotin label 12. Biotin labels RBC of all ages and is thus a “population” label rather than a “cohort” label. Seven of the current nine subjects (S1, S4–S9) had previous biotin label studies approximately five years prior to the stable isotope study (Table 1). Figure 2B shows a high correlation (R 2 = 0.79) between the M RBC values determined by the two methods, indicating agreement with respect to shorter vs. longer M RBC, even though the mode of labeling (ex vivo vs. in vivo ) and the type of label (population vs. cohort) are different.
Discussion
Important clinical decisions for millions of patients with diabetes rely on measurements of blood HbA1c. An implicit assumption is that for hematologically normal subjects RBC lifespan falls within a narrow range, and thus the association between HbA1c and plasma glucose concentration is the same for almost all subjects. The current study builds on the original stable isotope experiment reported by Shemin and Rittenberg in 1946 28 and subsequent studies 26, 27, 32 to demonstrate the potential usefulness of this method to evaluate variation in RBC lifespan. It combines with our earlier work utilizing the biotin labeling method 12 and studies using other methods 7, 13, 30, 31 to confirm important RBC lifespan variation in healthy subjects 12, and provides further evidence that RBC survival is likely to have an impact on HbA1c interpretation.
The stable isotope method has a number of attributes that make it well suited for at least clinical research measurement and possibly clinical assessment of RBC survival. M RBCdetermination could be useful for not only diabetes but also blood disorders such as sickle cell disease and the anemia of chronic inflammation. Compared to other RBC labels, it is relatively safe and convenient since there is no ex vivo RBC manipulation and no radioisotopes. Furthermore, all samples can undergo limited processing and freezing at the time of collection; this can then be followed by chemical extraction and mass spectrometry assayed for isotope ratio using a commercial laboratory in periodic large batches. This eliminates the need to perform inconvenient and expensive processing (e.g., flow cytometry on fresh cells) in real time throughout the RBC cell lifespan on each individual subject. Although multiple blood samples are required over a period of 4–6 months, the total blood volume needed is small. The stable isotope method is well‐suited for the study of modest changes in RBC lifespan since it measures the entire lifespan. However, studies of transfused RBCs can only be performed with ex vivo labels. Considering these potential applications of measuring RBC survival, an important question is whether the stable isotope method will reduce the cost and inconvenience of RBC survival measurements sufficiently to permit routine MRBC‐adjusted HbA1c determinations for the individual patient.
The high correlation between paired measurements of M RBC measured by the biotin label and stable isotope techniques approximately five years apart required both comparability of the two methods and stability of RBC lifespan with time. To our knowledge, there have not been prior publications providing direct evidence of stability of intra‐individual differences in M RBC over time. Our findings, however, are consistent with indirect evidence that M RBC is stable over a shorter time based on the constancy of HbA1c observed weekly over 3 months in persons without diabetes 10. We do observe, however, that the slope of the association between the two methods is not 1.0, and the intercept is not zero. This perhaps highlights some of the differences between the methods. For example, the cohort method measures both appearance and disappearance of the RBC age cohort. It is possible that assumptions made in normalizing the survival curve have systematically increased the estimated M RBC when compared with the biotin method, which relies on measurements of RBC disappearance only. We cannot rule out the possibility that the biotin labeling could also cause a subtle alteration that affects survival, although this is unlikely based on other studies that used this method 34.
The ability to measure M RBC in a large population has a number of implications. “J‐shaped” curves of mortality in relation to HbA1c have been described 35 revealing a paradoxical increase in mortality at the lower HbA1c ranges. Since HbA1c increases linearly with time in the circulation 12, its value must be dependent on both MBG and M RBC, and these paradoxical findings may result from differences in either M RBC or MBG. More generally, the availability of M RBC would permit evaluation of this variable for individualized glycemic management 36. The effect of RBC lifespan variability on assessment of glycemic control adequacy is evident from Fig. 3A demonstrating that at the extremes of possible RBC lifespan the same individual could be considered in the normal range for HbA1c or well above the threshold level of good glycemic control (7.0%). For Fig. 3A, the estimated average glucose (eAG) and corresponding HbA1c values were obtained from the A1c‐Derived Average Glucose (ADAG) trial 1 updated in 2013 37 in which blood glucose was measured by either self‐monitoring of blood glucose or continuous glucose monitoring over a period of ∼3 months and matched to HbA1c. Thus, secondary to M RBC variability, individuals with the same average blood sugar could have substantially different HbA1c levels. With a conservative estimate that variation in M RBC affects HbA1c interpretation in 10% of the people with diabetes in the United States, it is likely that glycemic control could be improved in substantially more than two million people in the United States alone by accounting for variation in RBC survival.
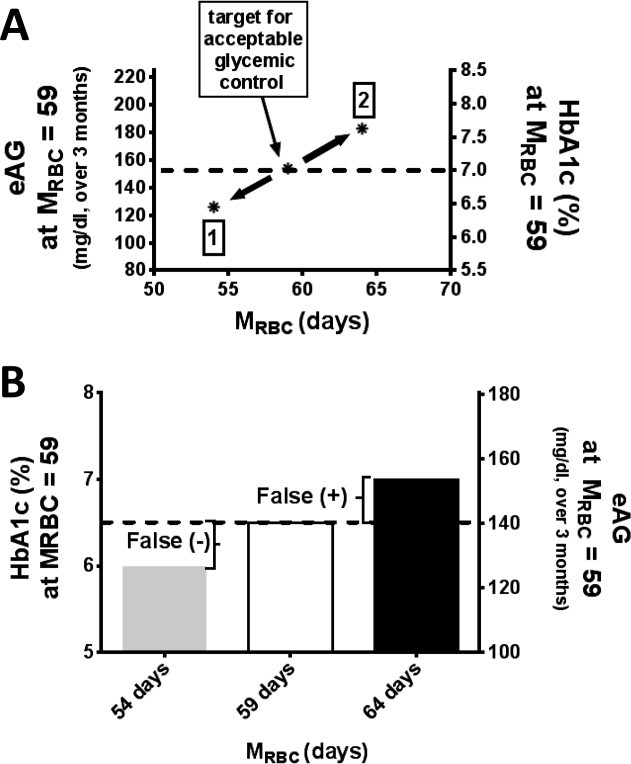
Figure 3
Open in figure viewerPowerPoint
The impact of RBC life span on HbA1c interpretation. A: Glycemic control resulting in HbA1c 7.0% at mean red cell survival (M RBC) 59 days would be expected to cause HbA1c as low as ∼6.2% (1) or as high as ∼7.6% (2) in people with diabetes and a range of M RBC 54–64 days (±15%, (2SD). The dotted line intersecting the right and left axes represents the estimated average glucose (eAG) and HbA1c respectively at an average M RBC of 59. B: The impact of accounting for M RBC (15% above and below the average) on HbA1c of 6.5%, the threshold for diabetes diagnosis. Below average and average M RBC would be expected to result in false negatives and false positives in the diagnosis of diabetes. The dotted line intersecting the right and left axes represents the eAG and HbA1c respectively at an average M RBC of 59.
Another potential application relates to the current low sensitivity (∼50%) of HbA1c for diagnosis of diabetes 38, 39. Increasingly, the diagnosis of diabetes is now being made at a specific HbA1c cut point, and the difference between values of 6.5% vs. 6.4% or 6.3% is being relied upon to inform an individual patient whether they do or do not have diabetes38. This decision was largely dependent on the ADAG study 1, which showed that subjects with normal hematocrits exhibit a strong average linear relationship between HbA1c and MBG. However, a closer analysis of those data demonstrates that there are wide confidence limits to that relationship, and our data would suggest that this is at least in part due to M RBC variability among people. Figure 3B demonstrates the implication of thisM RBC variation and supports the possibility that heterogeneity in M RBC partially explains the difficulty in finding a threshold HbA1c value with a high sensitivity for diagnosis of diabetes 15, 40.
Conclusions
In summary, there is considerable M RBC variation among hematologically normal individuals that is stable over time. As the noninvasive stable isotope method appears to be a feasible approach to measure RBC survival in relatively large populations, the opportunity now exists to assess the implications of M RBC variability for diabetes diagnosis and management.
Author Contributions
R.M.C., R.S.F., P.K.K., and E.P.S. conceived and designed the study, collected and compiled data, and wrote the manuscript. C.J.L. performed the calculations and statistical analysis and wrote the manuscript. S.M and S.H. helped design the study. M.C.R., M.B.P., and P.K.K. participated in the performance of the study including recruiting subjects and processing the blood samples. D.A.W. preformed the stable isotope measurements. J.M. H and C.H.J. helped conceive the study, interpret the data, and write the manuscript. All authors contributed to discussion and reviewed the manuscript. R.M.C is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Authors thank the participants in these studies and the nursing staff of the Clinical Research Unit at the Cincinnati VA Medical Center.
1 Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31: 1473– 1478.
2 Ismail‐Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010; 376: 419– 430.
3 Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577– 1589.
4 Nathan DM, Bayless M, Cleary P, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Advances and contributions. Diabetes 2013; 62: 3976– 3986.
5 Cohen RM, Lindsell CJ. When the blood glucose and the HbA(1c) don't match: Turning uncertainty into opportunity. Diabetes Care 2012; 35: 2421– 2423.
6 Bunn HF, Haney DN, Kamin S, et al. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest 1976; 57: 1652– 1659.
7 Virtue MA, Furne JK, Nuttall FQ, Levitt MD. Relationship between GHb concentration and erythrocyte survival determined from breath carbon monoxide concentration. Diabetes Care 2004; 27: 931– 935.
8 Garlick RL, Mazer JS, Higgins PJ, Bunn HF. Characterization of glycosylated hemoglobins. Relevance to monitoring of diabetic control and analysis of other proteins. J Clin Invest 1983; 71: 1062– 1072.
9 Smith RJ, Koenig RJ, Binnerts A, et al. Regulation of hemoglobin AIc formation in human erythrocytes in vitro. Effects of physiologic factors other than glucose. J Clin Invest 1982; 69: 1164– 1168.
10 Rohlfing C, Wiedmeyer H‐M, Little R, et al. Biological variation of glycohemoglobin. Clin Chem 2002; 48: 1116– 1118.
11 Mohás M, Kisfali P, Baricza E, et al. A polymorphism within the fructosamine‐3‐kinase gene is associated with HbA1c Levels and the onset of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2010; 118: 209– 212.
12 Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008; 112: 4284– 4291.
13 Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother 2012; 39: 302– 307.
14 Berlin NI, Waldmann TA, Weissman SM. Life span of red blood cell. Physiol Rev 1959; 39: 577– 616.
15 Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: Implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012; 97: 1– 6.
16 Khera PK, Joiner CH, Carruthers A, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 2008; 57: 2445– 2452.
17 Cohen RM, Joiner CH, Franco RS. Discordant HbA1c results: The hoofbeats increase. J Pediatr 2008; 153: 7– 9.
18 Franco RS. The measurement and importance of red cell survival. Am J Hematol 2009; 84: 109– 114.
19 Mock DM, Lankford GL, Widness JA, et al. Measurement of red cell survival using biotin‐labeled red cells: validation against 51Cr‐labeled red cells. Transfusion 1999; 39: 156– 162.
20 Franco RS, Lohmann J, Silberstein EB, et al. Time‐dependent changes in the density and hemoglobin F content of biotin‐labeled sickle cells. J Clin Invest 1998; 101: 2730– 2740.
21 Hibbert JM, Sutherland GB, Wright LL, et al. Measurement of hemoglobin synthesis rate in vivo using a stable isotope method. Anal Biochem 2001; 291: 118– 123.
22 Lindsell CJ, Franco RS, Smith EP, et al.: A method for the continuous calculation of the age of labeled red blood cells. Am J Hematol 2008; 83: 454– 457.
23 Egyed A. A simple method for heme isolation. Experientia 1972; 28: 1396– 1397.
24 Browne TR, Szabo GK, Ajami A, Wagner D. Performance of human mass balance/metabolite identification studies using stable isotope (13C, 15N) labeling and continuous‐flow isotope‐ratio mass spectrometry as an alternative to radioactive labeling methods. J Clin Pharmacol 1993; 33: 246– 252.



