ELEVATED EXHALED CARBON MONOXIDE CONCENTRATION…
ELEVATED EXHALED CARBON MONOXIDE CONCENTRATION IN HEMOGLOBINOPATHIES AND ITS RELATION TO RED BLOOD CELL TRANSFUSION THERAPY
Ellen Butensky James,Department of Gastroenterology, Children's Hospital & Research Center Oakland, Oakland, California, USA
Hendrik J. Vreman, Ronald J. Wong, David K. Stevenson, Department of Pediatrics, Stanford University School of Medicine, Stanford, California, USA
Elliott Vichinsky, Department of Hematology/Oncology, Children's Hospital & Research Center Oakland, Oakland, California, USA
Laurie Schumacher, Pediatric Clinical Research Center, Children's Hospital & Research Center Oakland, Oakland, California, USA
Judith Y. Hall, Department of Pediatrics, Spectrum Child Health, Stanford University, Stanford, California, USA
Julie Simon, Pediatric Clinical Research Center, Children's Hospital & Research Center Oakland, Oakland, California, USA
Daniel W. Golden and Paul Harmatz, Department of Gastroenterology, Children's Hospital & Research Center Oakland, Oakland, California, USA
In this study, the authors examined a possible role of measurements of end-tidal carbon monoxide (CO), corrected for inhaled CO (ETCOc), as a noninvasive screening tool for hemoglobinopathies and as an indicator for when transfusions would be required in patients receiving chronic transfusions. ETCOc measurements were obtained in subjects with sickle cell disease (n = 18), thalassemia (n = 21), and healthy controls (n = 62). ETCOc values less than 3 parts per million (ppm) yielded a positive predictive value of 93% and negative predictive value of 94% in identifying hemoglobinopathies. Subsequently, 7 subjects with thalassemia had laboratory parameters and ETCOc measured over 2 transfusion cycles. ETCOc values were 4.90 ± 0.32 ppm (mean ± SD), with 89% of values being above normal (≥3 ppm). Pretransfusion ETCOc levels significantly correlated with pretransfusion reticulocyte count (r = .96, P <.001), but not with pretransfusion hemoglobin (r = .44, P = .16) or pretransfusion soluble transferrin receptors (sTfR, r = .52, P = .10). In conclusion, we found that patients with hemoglobinopathies have ETCOc values above the range for healthy controls and ETCOc measurements can be used as an adjunct to hemoglobin measurements to determine the proper timing of transfusions.
Keywords : adults, children, end-tidal carbon monoxide, hemolysis, thalassemia, transfusion, sickle cell disease
Hemoglobinopathies are genetic disorders of hemoglobin structure (eg, sickle cell disease [SCD]) or production (eg, thalassemia) and affect more than 12.5 million people worldwide [l]. Common to both types of hemoglobinopathy is a decrease in the lifespan of red blood cells (RBCs) and a subsequent increase in the rate of heme turnover. Heme is oxidized, with one by-product being carbon monoxide (CO) , which is excreted with the exhaled breath. The concentration of CO in end-tidal breath, corrected for ambient CO (ETCOc) , can be measured and used as an index for the rate of heme degradation, RBC life span [2], or severity of a hemoglobinopathy [3,4].
A portable device used to measure ETCOc noninvasively (CO-Stat End Tidal Breath Analyzer ; Natus Medical, San Carlos, CA) was previously used to demonstrate that endogenous CO production rates are elevated in patients with SCD or thalassemia [4].
The aims of this study were to determine if noninvasive ETCOc measure ments could be used as a screening tool to identify patients with possible hemolytic or sideroblastic hemoglobinopathies and as a monitoring tool to assess the severity of ineffective erythropoiesis and, therefore, serve as an adjunct to hemoglobin (Hb) to direct transfusion therapy in patients with thalassemia.
MATERIALS AND METHODS
Study Design
Patients were enrolled at Children’s Hospital & Research Center Oak land under the auspices of an Institutional Review Board-approved protocol that included informed consent.
Cross-Sectional Study
A convenience sample of 101 children (2 to 20 years of age) and adults (≧21 years of age), who were healthy or diagnosed with SCD or thalassemia,were eligible for the cross-sectional study. A total of 58 children were en rolled , including 27 (46%) healthy controls, 15 (26%) with thalassemia, and16 (28%) with SCD. A total of 43 adults were enrolled, 35 (81%) healthy controls, 6 (14%) with thalassemia, and 2 (5%) with SCD. Primary smokers were excluded. Ten (9 with thalassemia, 1 with SCD) of the subjects were receiving routine RBC transfusion therapy during the study.
Subjects with SCD or thalassemia had Hb levels and reticulocyte counts measured as part of their routine clinical care. Hemoglobin levels were measured using a Coulter counter (Coulter Electronics, Luton, UK). Reticu -locyte counts were determined using flow cytometry (Sysmex Corp. of Amer -ica, Long Grove, IL). ETCOc concentrations, expressed as parts per million (ppm) were measured using the CO-Stat. All subjects had ETCOc concen tration measured on at least one occasion. In patients receiving routine transfusion therapy, ETCOc measurements were obtained 2 to 47 days post transfusion (mean ± SD: 18.4 ± 13.4 days).
Longitudinal Study
Children and adults with thalassemia or RBC aplasia receiving chronic RBC transfusions were deemed eligible for the longitudinal phase of the study. Patients who were primarγ smokers or taking hydroxyurea or rib avirin at the time of the study were excluded due to effects on ET COc and background hemolysis. As a result, 7 subjects (3 females, 4 males) ranging in age from 8 months to 27 years (16.1 ± 8.9 years) with thalassemia were studied. Primarγ diagnosis of the subjects in -cluded .B-thalassemia in 6, E/.B-thalassemia in 1. One additional patient, a 14 year-old male with Aase’s disease (RBC aplasia) on regular transfu sion therapy, was included to se凹e as a control for breakdown of circu lating RBCs derived from transfused blood in the absence of ineffective erythropoiesis. This subject was not included in the analysis of subjects with hemoglobinopathies.
Subjects were followed through 2 routine RBC transfusion cycles. ETCOc measurements were obtained immediately pretransfusion and at approxi mately 1 to 2, 7, and 14 days post-transfusion. At each time point, ETCOc measurements were made 1 to 3 times with each patient at the same loca tion. When more than one sample measurement was made, the mean value was used for data analysis. Soluble transferrin receptor (sTfR) and retic ulocyte count, as indicators of bone marrow hyperplasia, were measured along with complete blood count pre- and 1 to 2 days post-transfusion. sTfR were analyzed by enzyme immunoassay (Specialty Labs, Santa Monica, CA).Hemoglobin levels and reticulocyte counts were performed as described above.
Statistical Analysis
Data are presented as mean ± SD. Linear regression and the Student’s t test analyses were used to assess the relationship between laboratory param-eters. Sensitivity, specificity, and a receiver operating characteristic curve for ETCOc were analyzed with Analyse-It (Analyse-It Software, Ltd., Leeds, UK). All other analyses were performed using analysis of variance (ANOVA). Significance was set at P < .05.
RESU LTS
Cross-Sectional Study
Laboratory data were available for 23 subjects with SCD or thalassemia. Of these data, 6 results were collected more than 2 days before or after the ETCOc measurement and, therefore, were not included in the analysis. The results for the remaining 17 subjects are presented in Table 1.
There was no significant difference between the thalassemia group (n= 14) and the SCD group (n=3) in Hb concentration (thalassemia: 9.97± 2.03 g/dL versus SCD: 8.10 ± 1.61 g/dL, respectively, P = .16) or retic ulocyte count (thalassemia: 2.20 ± 1.90% versus SCD: 9.63 ± 4.35%, re spectively, P = .09). When the groups were grouped by transfusion status, transfused subjects with thalassemia demonstrated significantly higher Hb concentrations and significandy lower reticulocyte counts. (This analysis could not be performed in the SCD group due to small sample size; see Table 1.)
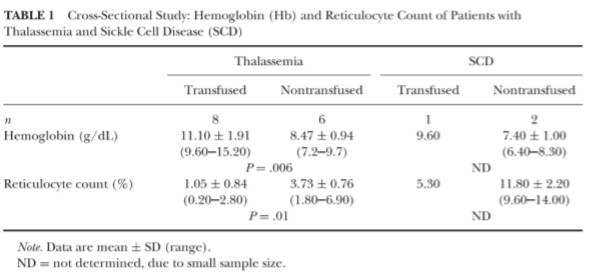
ETCOc values are presented by age and diagnosis in Table 2. ETCOc levels were found to be statistically different between all groups (P < .006) , with the highest values seen in the SCD group and the lowest values in the control group. Of the 43 adults, ETCOc levels were found to be significandy different (P 二 .001) between the control group (1.8 ± 0.7 ppm) and the thalassemia group (5.8 ± 1.5 ppm) only, although there were only 2 subjects in the adult SCD group.
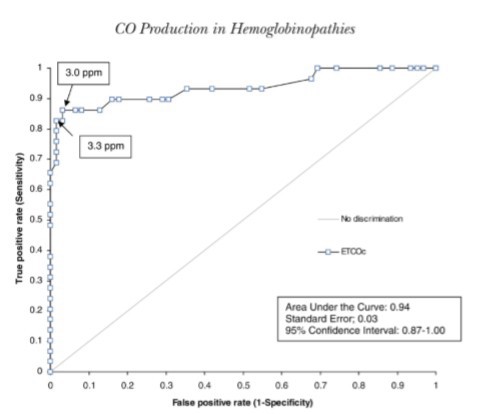
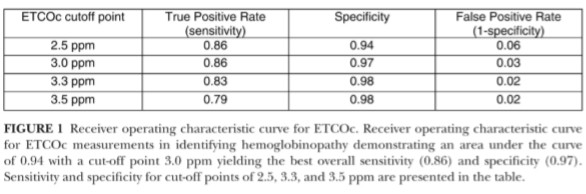
Applying a value of <3.0 ppm as the cut-off for normal values, ETCOc measurements had a sensitivity of 0.86 and a specificity of 0.97. This cut-off point yielded the highest overall sensitivity and specificity when compared to cut-off values of 2.0, 3.0, and 3.5 ppm, as demonstrated in Figure 1. The area under the curve for the receiver operating characteristic curve for ETCOc was 0.94.
Longitudinal Study
No patient was an active smoker, although the control subject with Aase's disease was exposed to second-hand smoke in the home. Because the in fant (an 8-month-old female with . β-thalassemia) was heavily transfused, and demonstrated complete suppression of erythropoiesis, as determined by pre transfusion Hb of 11.3 g/dL and a normal sTfR concentration, she was ex eluded from the analysis. The mean age of the 6 remaining subjects was 19.2 ± 5.1years (range: 13 to 27 years).
Subjects were followed for a range of 5 to 8visits per patient. Hb and sTfR measurements were categorized as either pre- or post-transfusion. Mean pre and post-transfusion ETCOc levels were not statistically different (5.6 ± 2.6 and 5.6 ± 3.0 ppm, respectively). Pretransfusion sTfR significantly correlated with pretransfusion Hb levels ( r = .70, P = .02) and with pretransfusion reticulocyte counts (r = .82, P= .002). Mean ETCOc, as determined by the mean of all measurements taken at one study time point, ranged from 2.0 to 13.5 ppm (mean ± SD: 4.9 ± 0.3 ppm). Eighty-nine percent of the ETCOc measurements in the thalassemic subjects were above the normal range. Four of the six subjects with thalassemia had no ETCOc value within the normal range (<3 ppm) . Pretransfusion, ETCOc concentrations correlated signi白cantly with reticulocyte count (r = .96, P < .001) , but not with sTfR (r = .52, P= .10) or Hb (r = .44, P = .16) levels.
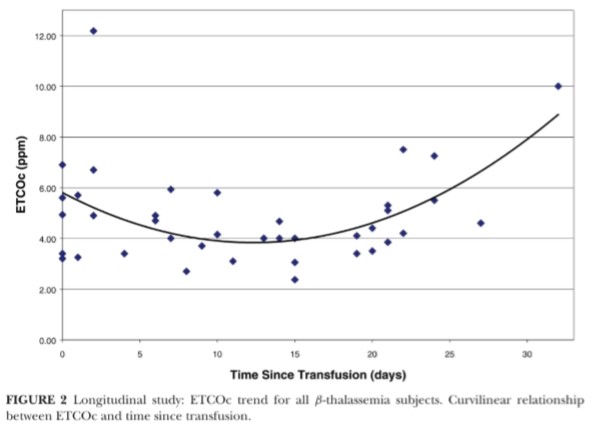
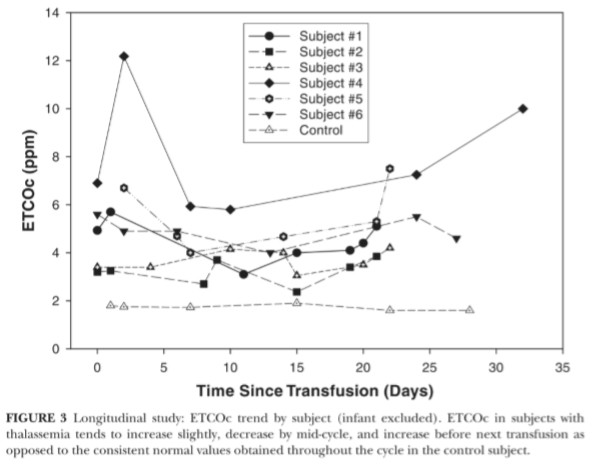
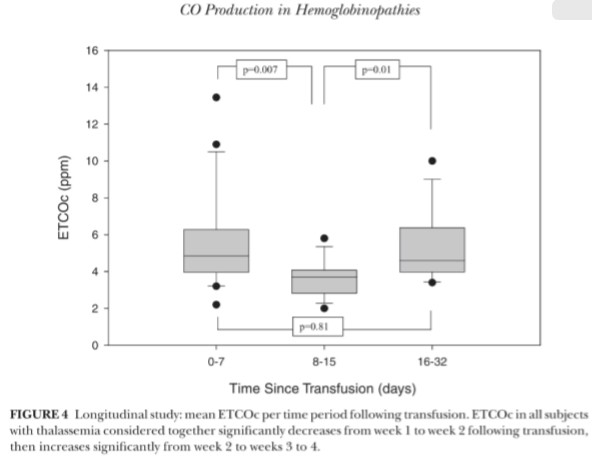
There was no significant linear relationship between “time since trans fusion” and ETCOc levels ( r 二 .01, P 二 .94) , therefore, a curvilinear re lationship was explored (Figure 2) . Transfusion cycles were found to be independent of one another (Pearson r = -.16, P= .22); therefore, ETCOc values for the 2 cycles were combined for each individual. ETCOc measurements had an overall trend to peak after transfusion, then dip 2 weeks post-transfusion, and increase over time to the next transfusion (Fig ure 3) . ETCOc values were grouped by “time since transfusion" (period 1: 0 to 7 days; period 2: 8 to 15 days; and period 3: 16 to 32 days). The mean ETCOc value dropped significantly from time period 1to 2 and then signif-icantly rose again at period 3 (Figure 4). Periods 1 and 3 were not signify-cantly different. The mean of the low ETCOc measurements for each subject (3.8 ± 1.2 ppm) were significantly lower than the mean of the pretransfu sion ETCOc measurements (5.1 ± 2.1 ppm, P = .01) for the first transfusion cycle. This comparison could not be made for the second transfusion cycle due to missing values.
DISCUSSION
Subjects with SCD and thalassemia demonstrated higher ETCOc con centrations than healthy controls and confirmed findings reported by others [5]. The high sensitivity and specificity (using <3.0 ppm as a cut-off for the normal range) support the potential of using ETCOc as a non invasive, rapid method to screen for populations with increased hemoly-sis, including populations with hemoglobinopathies. Routine screening for hemoglobinopathies using biochemical testing methods is presently prac -ticed as part of newborn blood screening program in 45 states. These meth -ods have positive and negative predictive values of 99% and 100%, respect-tively [6]. Although biochemical testing requires phlebotomy and accurate reporting of results to families or physicians, it has the advantage of provid -ing diagnostic direction prior to onset of disease complications. In this study, ETCOc measurements were found to have lower, yet reasonable, positive and negative predictive values for a screening tool, but ETCOc monitoring is a method that is noninvasive , and provides immediate results. Therefore, the use of ETCOc measurements may be a viable option for “first-stage” screening in states or countries that do not currently conduct newborn screening for hemoglobinopathies. Confirmation would require specific testing for hemoglobinopathies, as other causes of hemolysis including infection, en zyme or membrane defects, and nutritional deficiencies could also increase the CO. It will, however, be necessary to determine the optimal and earliest age at which such “first-stage ” screening should be instituted. The CO-Stat is no longer commercially available. However, alternative devices that can be used for these purposes have been developed , including the COC02 Puff (Everest Biomedical Instruments, Chesterfield, MO) .
Failure to observe an anticipated correlation between ETCOc and “time since transfusion" can be explained by the finding in the longitudinal study that the ETCOc levels measured between transfusions were not a linear function of time. In fact, a curvilinear relationship was found and may reflect the initial suppression of ineffective erythropoiesis and hemolysis following transfusion, which wanes over time.
sTfR, sampled 1 to 2 days after transfusion, had not changed from pre transfusion levels, suggesting that this delay did not allow sufficient time for down-regulation of the transferrin receptor (TFRC) gene. Supporting this interpretation is the demonstration of a drop in sTfR by 50% from pretrans fusion levels (and the lowest value observed in the . β-thalassemia subjects) in one subject from whom sTfR was obtained 4 days post-transfusion. Further evaluation of the sTfR data, particularly those in mid-cycle between transfu sions, should be performed and is likely to reveal the nadir of bone marrow hyperplasia, hopefully in the normal range. However, multiple blood sam plings between transfusions in individual patients as a clinical procedure would be difficult and undesirable. Alternatively, rapid and noninvasive ETCOc measurements can be used to assess increased heme breakdown from hyperplasia in the bone marrow in this population.
Pretransfusion ETCOc levels correlated with reticulocyte counts and are an indicator of bone marrow ineffective erythropoiesis. There was no cor relation between pretransfusion ETCOc and sTfR levels, which may reflect the small study group size and the large interindividual variation in sTfR and ETCOc values. In contrast, when each individual was followed over time, a consistent pattern emerged. There was an increase in ETCOc lev els shortly after transfusion, reflecting a combination of continuing inef fective erythropoiesis and heme breakdown from the increased circulating RBC volume, followed by a decline, which mirrors the decline in sTfR ob served by others [7], reflecting a suppression of eηthropoiesis and decrease in circulating RBC mass. At the end of the cycle, there was another in crease in ETCOc levels, as bone marrow suppression waned, to values significantly higher than the nadir values despite continued decrease in circulating RBC mass.
From this study, it appears feasible to use ETCOc measurements as a first-stage” screen for hemolysis, including hemoglobinopathies. In addi tion, we suggest that, in thalassemia, expired CO can be used as an adjunct with hemoglobin to identify increased bone marrow hyperplasia and the need for transfusion.
ACKNOWLEDGMENTS
The study was supported by Natus Medical, Inc., San Carlos, CA, and by the National Institutes of Health, contract grant numbers M01-RR00070 (Stanford University), M01-RR01271-16, HL-20985, and MCJ-061016-06 (Children’s Hospital & Research Center Oakland) .
Declaration of Interest: The authors report no conflicts of interest. Ju dith Hall was employed by Natus Medical, Inc. and Hendrik J. Vreman, Ronald J. Wong, David K. Stevenson were consultants to Natus Medical, Inc. at the time the study was performed. The authors alone are responsible for the content and writing of the paper.
REFERENCES
[1] Vichinsky E. The future of thalassemia treatment: introduction. Int J Pediatr Hematol/Oncol.
1997;4:l-2.
[2] Strocchi A, Schwartz S, Ellefson M, eta!.A simple carbon monoxide breath test to estimate erythrocyte turnover.] Lab Clin Med. 1992;120:392-399
[3] Vreman HJ, Baxter LM, Stone RT, et al. Evaluation of a fully automated end-tidal carbon monoxide instrument for breath analysis. Clin Chem. 1996;42:50-56.
[4] Vreman HJ,Wong RJ,Harmatz P, et al. Validation of the Natus CO-Stat End Tidal Breath Analyzer
in children and adults.J Clin Monit Comput. 1999;15:421-427.
[5] Chan GC, Lau YL, Yeung CY. End tidal carbon monoxide concentration in childhood haemolyuc disorders.] Pα,ediatr Child Health. 1998;34:447-450.
[6] Papadea C, Eckman JR, Kuehnert RS, et al. Comparison of liquid and dried blood for neonatal hemoglobinopathy screening: laboratory and programmatic issues. Pediatrics. 1994;93:427-432.
[7] Cazzola M, Borgna-Pignatti C, Locatelli F, et al. A moderate transfusion regimen may reduce iron loading in beta-thalassemia major without producing excessive expansion of e,γthropo iesis. Transfu-sion.1997;37:135-140.



